Control of Protein Phosphatases
Mitotic exit is enforced by phosphatases that remove the phosphate that mitotic kinases put on targets in order to drive the re-arrangements of cellular architecture that generated the mitotic apparatus. Just as the sequence of phosphorylation events that drives spindle formation must be highly co-ordinated, the removal of these phosphates must be equally well controlled to ensure the staged progression from one phase of division to the next.
Over 95% of phosphatase activity human cells has been attributed to Protein Phosphatase 1 (PP1) and Protein Phosphatase 2A (PP2A). PP1 acts as a monomer that is recruited to targets and platforms from which it can dephosphorylate substrates. In contrast, PP2A is trimeric with catalytic, structural and regulatory subunits. Of the four types of regulatory subunit, PP2A holoenzymes containing the B55 and B56 isoforms have been linked to mitotic exit. The direct inhibition of PP1 by Cdk1-Cyclin B has long highlighted the potential of this phosphatase for mitotic control, while functional studies identified a key role for PP2A phosphatases in driving mitotic exit. Because the recruitment of PP1 to specific sites imparts specificity upon PP1 and the specificity of PP2A phosphatase has been attributed to the specific interface generated between the juxtaposition of a specific regulatory subunit and the catalytic subunit, PP1, PP2A-B55 and PP2A-B56 have been assumed to play a distinct and specific roles in mitotic exit. We were therefore surprised to see direct and functional dialogue between PP1 and PP2A.
We found that the staged recruitment of PP1 to docking sites in the regulatory subunits of the PP2A-B55 and PP2A-B56 phosphatases drove the sequential activation of the PP2A enzymes. The activity of all three phosphatases is repressed upon mitotic commitment. It is currently unclear which phosphorylation events repress PP2A-B55 or PP2A-B56 activity. PP1 activity is repressed by Cdk1-Cyclin B phosphorylation. The enzyme that removes this inhibitory phosphate from PP1 is PP1 itself. Thus, Cyclin B destruction automatically supports the reactivation of PP1 and any PP2A enzyme to which PP1 can be recruited. PP1 binds to PP2A-B55 from mitotic commitment to genome segregation in anaphase. Because Cyclin B destruction enables PP1 to auto-catalytically remove the Cdk1-Cyclin B imposed inhibitory phosphorylation, it also supported the reactivation of PP2A-B55 by PP1.
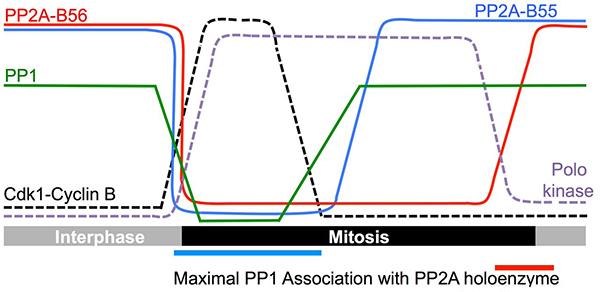
Although PP1 can bind and re-activate the B55 regulatory subunit when Cyclin B is destroyed, PP1 binding to the B56 regulatory subunit is blocked by phosphorylation within the PP1 docking site by the mitotic protein kinase PoloPlo1. This phosphate must be removed before PP1 can be recruited to restore PP2A-B56 activity. The enzyme responsible for removing this phosphate is PP2A-B55. Thus, an ordered sequence of events follows Cyclin B destruction with the first being the auto-reactivation of PP1. As PP1 can bind PP2A-B55 at this time, PP2A-B55 reactivation is coincident with PP1 reactivation. This newly reactivated PP2A-B55 then tries to dephosphorylate B56, however PoloPlo1 phosphorylation of B56 is more efficient than PP2A-B55 de-phosphorylation. PP1 is therefore unable to bind and re-activate PP2A-B56 until PoloPlo1 activity declines at the end of mitosis. Thus, PP1 acts as a master controller in a phosphatase relay to control the de-phosphorylation events that are required to drive cells out of mitosis.
An engaging collaboration with Anja Hagting and Jon Pines in the Gurdon institute in Cambridge, established that human PP1 binds the docking site of human PP2A-B56 to suggest that the core principles of the phosphatase relay we have identified in fission yeast are conserved in human cells.
We believe that the sequential nature of this phosphatase relay will only be true in in vitro biochemical reactions. We anticipate that the specific concentration of each component of this relay at any given location will determine which activity predominates at this site in vivo. Thus, full PP2A-B56 activity may be supported at one discrete location, while the remaining enzyme throughout the rest of the cell is repressed.