Regulation of Cell Morphogenesis
The small GTPase Cdc42 plays a key role in regulating the activity of a number of actin mediated events to control morphogenesis in all eukaryotes. GTP Exchange Factors (GEFs) promote the exchange of GTP for GDP to generate the active Cdc42GTP that stimulates effectors, including PAK kinases. The complete reliance of S. pombe upon Cdc42 for growth by linear extension makes it an excellent model with which to study Cdc42 control. Cell division by medial fission generates daughters that each grow from the mature tip that grew in the previous cell cycle. The new end that was generated by cytokinesis in the preceding cell cycle remains dormant until activation of PoloPlo1 kinase at the spindle pole body (SPB) triggers New End Take Off (NETO) in late G2 phase.
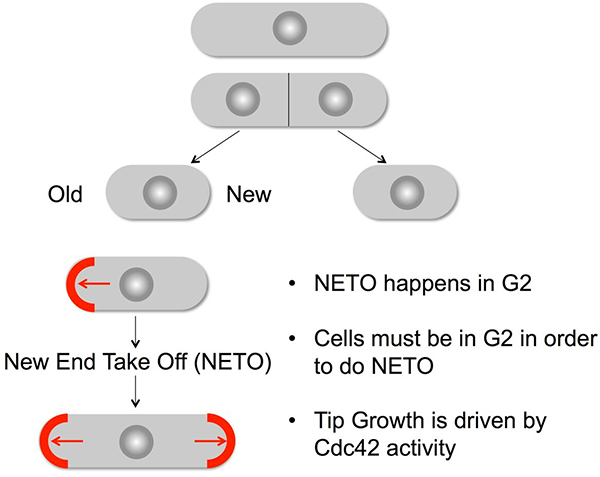
The time at which Cdk1Cdc2-Cyclin B activation at the G2 SPB initiates the polo kinase dependent feedback loop that triggers NETO is determined by the recruitment of protein phosphatase 1 (PP1) to the SPB component Cut12. Mutations, such as cut12-s11, that compromise PP1 recruitment both advance the point in G2 phase at which the loop is initiated to boost global levels of PoloPlo1 kinase activity and advance the time in G2 at which NETO is triggered. There is a direct correlation between the level of PP1 recruitment to Cut12 and the activity of PoloPlo1, both locally at the SPB and globally throughout the cell. This relationship extends to a direct relationship between the level of PoloPlo1 activity and the timing of NETO. The sooner PoloPlo1 is activated, the sooner NETO is triggered. The synthetic biology approach of releasing PoloPlo1 activity at discrete sites around the cell indicated that NETO is only triggered when PoloPlo1 activity is released at the SPB. PoloPlo1activation at the SPB also elevated the density of F-actin cytoskeleton throughout the cell.
We are therefore using global phosphoproteomic approaches to compare the phospho-proteome of cells with elevated or reduced PoloPlo1 signalling in an attempt to identify the pathway by which PoloPlo1 activation at the SPB regulates cell cycle dependent morphogenesis several microns away at the cell tip. As phosphorylation of PoloPlo1 on serine 402 is required for appropriate cell tip growth after recovery from heat/centrifugation stress, we are using this assay to tease out which aspects of Polo kinase function are associated with cell cycle dependent morphogenesis and which with stress recovery morphogenesis.