Mitotic Spindle
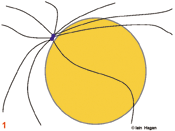
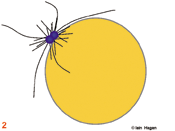
The duplicated genome is segregated into two daughter cells through the action of a complex structure which forms upon commitment to cell division called the mitotic spindle. Spindle structure and function can be viewed in terms of the organisation of its major structural components, the filamentous protein polymers called microtubules. During interphase the microtubule cytoskeleton is differentiated to suit the needs of the particular cell type. Generally speaking microtubules are nucleated from a juxtanuclear microtubule orgranising centre (MTOC), the centrosome in metazoans (purple spheres). The centrosome duplicates in G1 phase of the cell cycle so that in actively cycling cells the G2 organiser is composed of two side by side centrosomes (1). Upon commitment to mitosis the centrosome matures by a mechanism that involves NIMA and Polo related kinases. It becomes enlarged and nucleates more microtubules (2). At the same time microtubule dynamics and distribution undergo radical changes as the interphase microtubule array disappears and microtubules extend from the enlarged centrosomes to probe the 3-D space around each centrosome to set up the short prophase spindle (3). As the chromosomes condense in prophase, the MTOCs migrate around the surface of the nucleus (3). Microtubule motor proteins that favour the anti-parallel association of microtubules interact with those microtubules that extend into the region between the MTOCs to promote their interdigitation and so form the early spindle. The nuclear envelope (grey line in 1-3) breaks down and chromosomes are exposed to the probing microtubules from the two spindle poles (4). The break down of the nuclear envelope marks the end of antephase . During antephase cells are able to respond to stresses such as DNA damage and exit mitosis, repair the damage and then re-commit to mitosis when the damage has been repaired.

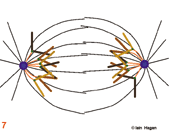
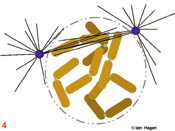
The spindle assumes a characteristic form as some microtubules are bound by specialised chromosomal domains called kinetochores (green dots – the red lines in 5 - 8 show kinetochore microtubules). Attached kinetochores re-orientate to face the MTOC from which the interacting microtubules extend. One kinetochore attaches to the microtubules extending from one pole whilst its sister attaches to those extending from the other. If a kinetochore is not attached to microtubules it activates the spindle assembly checkpoint system that blocks the subsequent stage of anaphase by blocking the activity of the anaphase promoting complex that destroys securin and cyclin B . The intensity of the green kinetochore signal in the figures indicates checkpoint status, lightest, both unattached, intermediate, one attached, darkest both attached. Once all of the kinetochores have been captured by microtubules, the chromosomes align mid-way between the two poles (metaphase) and the kinetochore generated checkpoint signal is no longer produced (6).
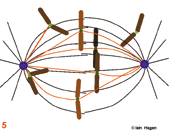
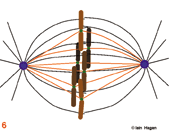
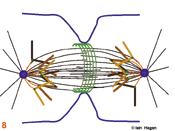
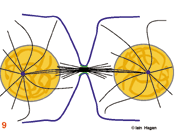
Eventually the checkpoint signal drops below a threshold and no longer inhibits commitment to anaphase in which the chromosomes split into two chromatids that move to the poles (7). During the final stage, telophase, the acto-myosin cleavage furrow (green lines in 8 & 9) constricts to bring about cytokinesis (9). As the furrow constricts spindle morphology changes. The central bundle becomes stronger as microtubules emanate from an organelle that forms as this site called the mid body . A nuclear envelope re-forms around the de-condensing chromosomes, an interphase microtubule array is re-established in each daughter cell and the cell resumes its specialised role (9).
These elegant chromosome movements are achieved by a complex interplay of microtubule dynamics, microtubule motor proteins that are capable of moving substrates along microtubules, and checkpoint controls.