Overview
Since its conception, the Cancer Inflammation and Immunity group has been investigating the mechanisms underlying natural and therapy-induced tumour immunity. A special focus has been the identification of the instructive signals that, by regulating the balance between tumour-promoting and tumour-suppressive inflammation, favour immune escape and therapy resistance (Figure 1 below). Our overarching hypotheses are that the inflammatory landscape at the tumour bed is a key determinant of patient outcome, and that the pro-tumourigenic properties of classic cancer-related inflammation are largely ascribed to its inhibitory effects on the anti-cancer functions of the immune system.
Our leading questions are:
- What triggers and sustains the immune response against cancer?
- What are the mechanisms by which tumour cells manipulate their environment and evade immune control?
- Can we leverage information about the inflammatory makeup of a tumour to better distinguish responders from non-responders?
- Can we innovate treatment strategies to convert treatment refractory tumours into responsive to immunotherapy?
To address these questions, we are using versatile pre-clinical in vitro and in vivo models of cancer. Genetic and pharmacological manipulation coupled to cutting-edge immune-profiling and omics approaches support detailed mechanistic understanding and establishing causality. In addition, we analyse patient samples from publicly available datasets and from unique Manchester cancer patient cohorts to place our findings in a physiologically relevant human disease context for clinical translation. This multidisciplinary approach combining analysis of preclinical models and clinical samples is key to deepen our knowledge of cancer biology, to develop biomarkers that predict patient outcomes and to design more efficacious and safer interventions.
Under the premise that further fundamental research is required to make immunotherapies better and safer, my group investigates the principles that determine the susceptibility of tumours to spontaneous or therapy-induced cancer immunity. Our central hypothesis is that the inflammatory landscape at the tumour bed, in both early and late cancer stages, before and post-treatment, critically influences tumour fate. However, cancer-associated inflammation is a complex process that manifests in different ‘flavours’ with antagonistic tumour-promoting or -inhibitory effects. Within this working model, and a rapidly evolving clinical scenario, our current research objectives are:
- To deepen our understanding of the mechanisms that regulate the establishment of tumour inflammatory environments that either stimulate or hinder immune-mediated tumour control,
- To develop approaches to better predict patient outcome and guide treatment selection,
- To identify translatable therapeutic strategies to manipulate intratumoural inflammation and improve the efficacy of immunotherapy.
Featured Publications
Glucocorticoids Unleash Immune-dependent Melanoma Control through Inhibition of the GARP/TGF β Axis
15th October 2025
This study uncovers a surprising role for GCs in triggering CD8+ T cell–dependent tumour control through downregulation of GARP and thus TGF β signalling.
Chemotherapy-induced COX-2 upregulation by cancer cells defines their inflammatory properties and limits the efficacy of chemoimmunotherapy combinations
19th April 2022
Cytotoxic therapies, besides directly provoking cancer cell death, can also stimulate immune-dependent tumour growth control or paradoxically accelerate tumour progression. Here, the authors show that all chemotherapy drugs acutely upregulate COX-2 and PGE2 production in cancer cells with pre-existing COX-2 activity and, in doing so, fuel cancer immune escape post-treatment.
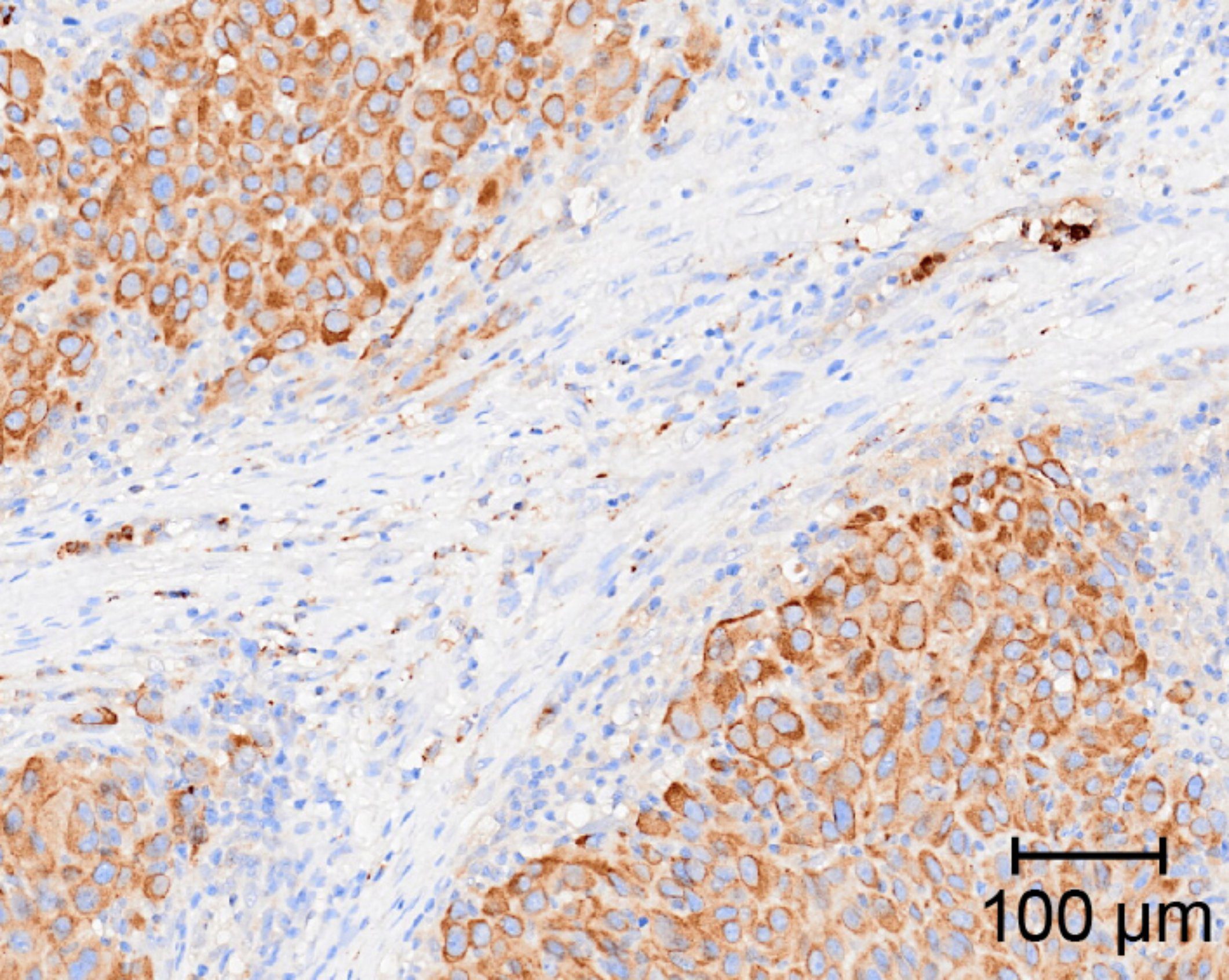
Anti-Inflammatory Drugs Remodel the Tumor Immune Environment to Enhance Immune Checkpoint Blockade Efficacy
11th October 2021
Through in-depth cellular and molecular profiling of mice and human tumours, the authors identify the mechanisms by which anti-inflammatory drugs targeting the COX-2/PGE2/EP2-4 axis rapidly alter the tumour immune landscape to enhance the response to immune checkpoint inhibition.
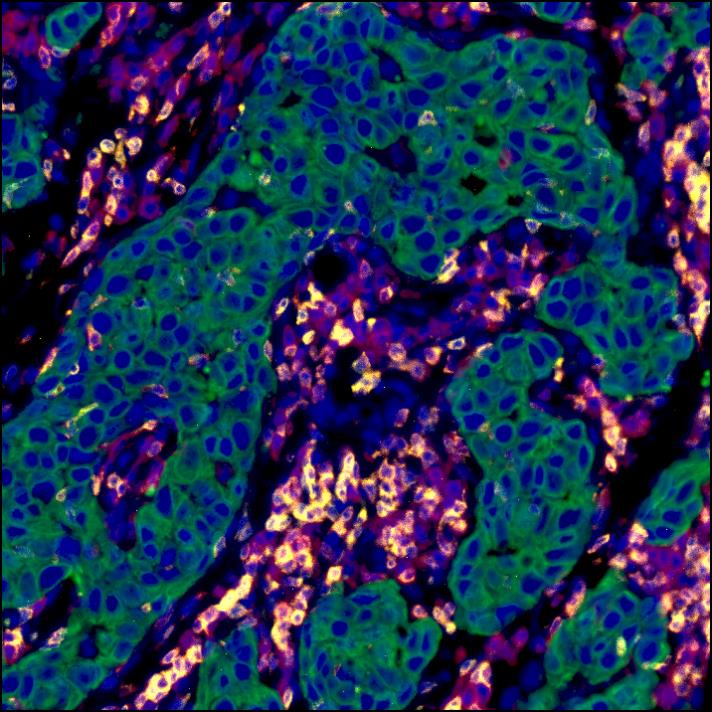
Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade
15th December 2020
The authors demonstrate that cancer cell-derived PGE2, acting on EP2 and EP4 on NK cells, restrains T cell-dependent tumour control and devise a COX-2-associated inflammatory signature that predicts patient survival and response to immunotherapy.
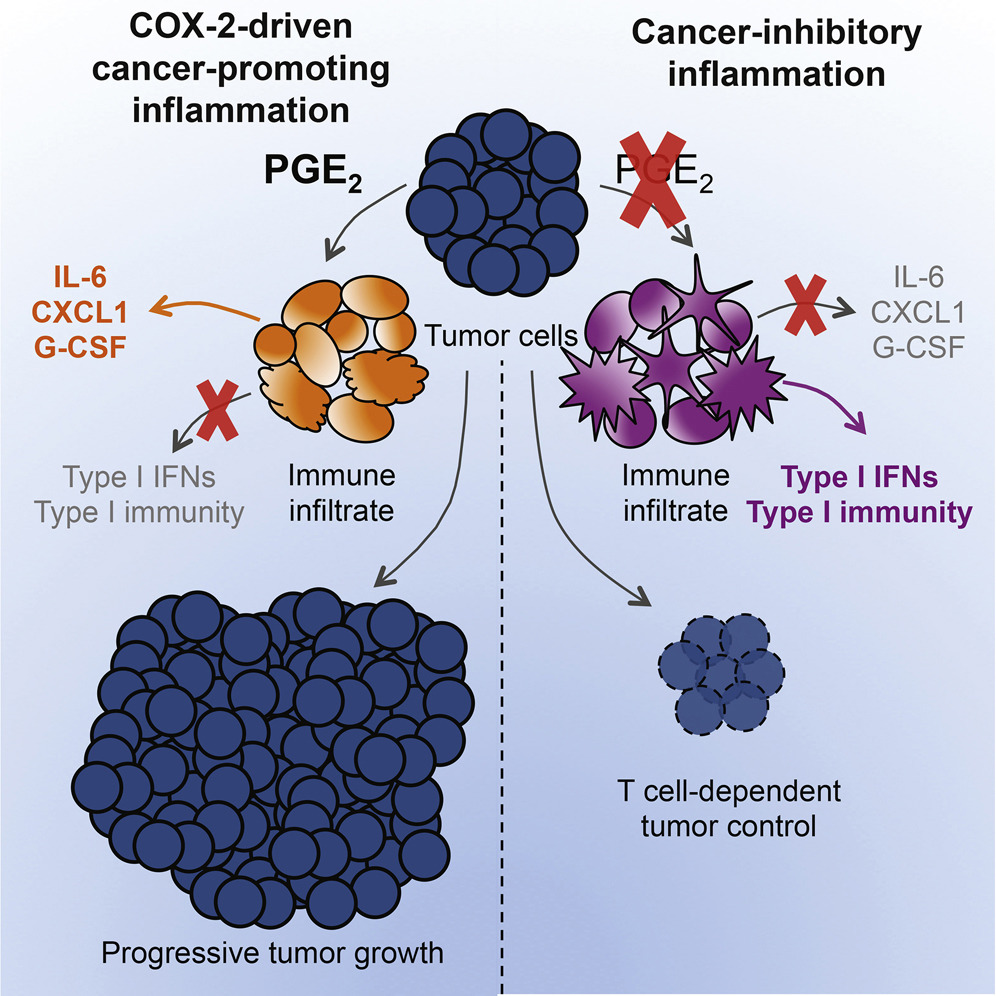
Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity
3rd September 2015
The COX-2/PGE2 pathway is commonly upregulated in multiple tumours. Here, the authors show that this inflammatory axis drives malignant growth by enabling immune escape and that cyclooxygenase inhibitors synergise with immunotherapy to promote tumour eradication.
Our working hypothesis
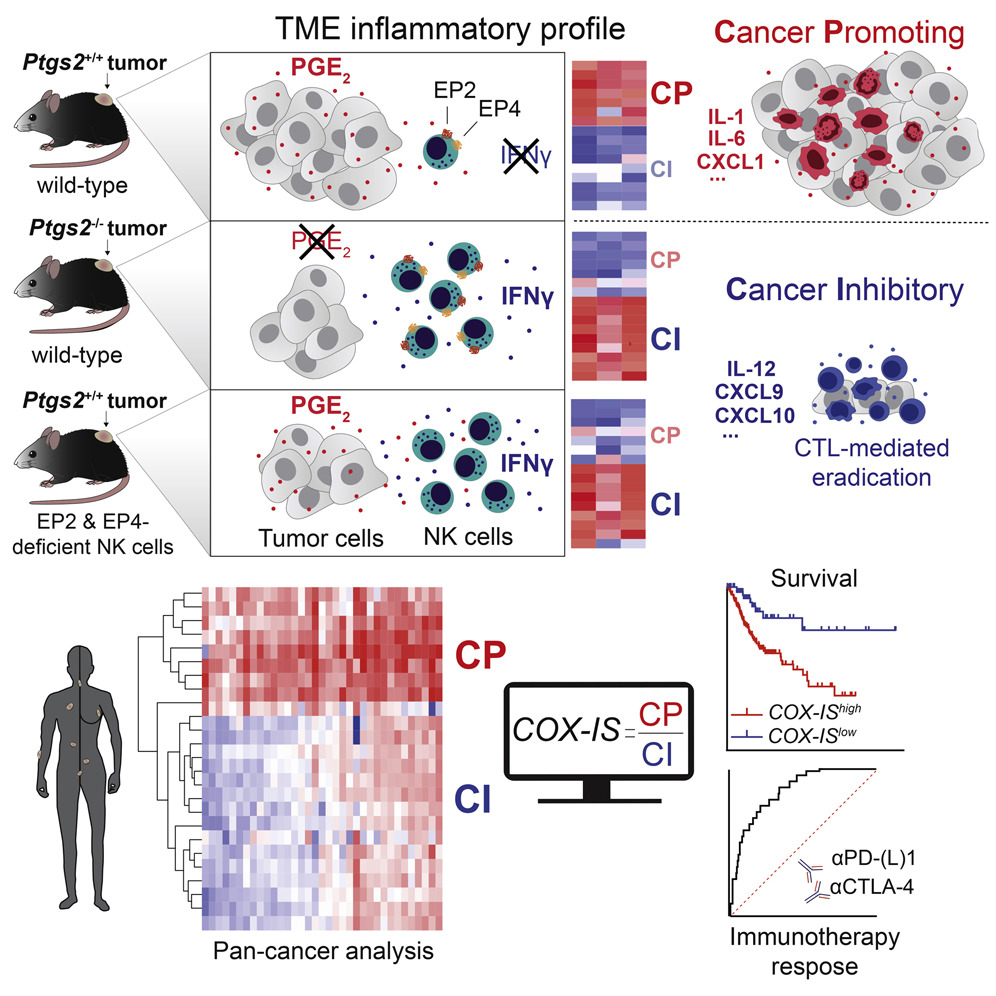
Tumour fate and outcome from therapy depend on the nature of the inflammatory response at the tumour bed.
The figure to the right includes key research interrogation points directed at identifying the mechanisms that control spontaneous and therapy-induced tumour immunity, focused on the cellular and molecular mediators that regulate the balance between cancer-promoting and cancer-inhibitory inflammation. The snake of the Greek god of medicine Asclepius, winding around and tipping the balance towards cancer-inhibitory inflammation represents our search for pharmacological interventions to improve the efficacy of cancer therapies that harness the anti-cancer functions of the immune system.
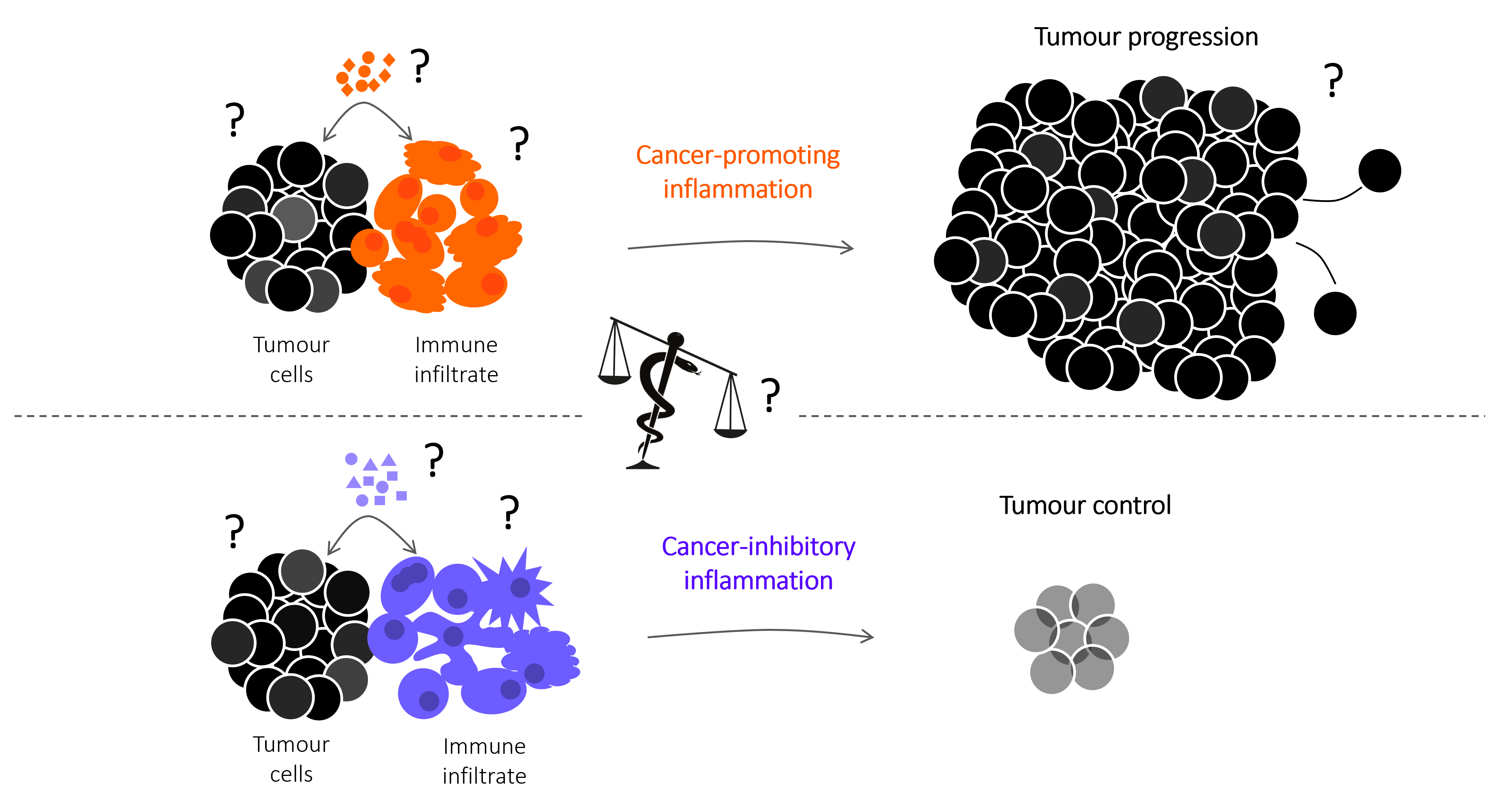
The dual role of inflammation in cancer immunity
Inflammation can fuel or limit cancer growth and the response to therapy. Our group have revealed an intimate link between the COX-2/PGE2 pathway, NK cells and cytotoxic immunity that defines the intratumoral inflammatory composition and anticipates tumour fate. This illustration depicts the complex Yin and Yang interaction between pro- (yellow) and anti-tumorigenic (blue) inflammatory responses within a tumour. By in-depth profiling of murine cancer models and bioinformatics analysis of cancer patient datasets, we devised a gene-expression signature that predicts overall patient survival and response to immunotherapy across multiple cancer types (in the background) with varying degrees of opposing inflammatory milieus. Illustration by Sam Falconer.

Join our group
We are currently looking for a Computational Immunologist in our lab – please go to vacancy:
https://www.cruk.manchester.ac.uk/career/mi-25-17_2-computational-immunologist/
Or visit our Careers page.
Get in touch
All Institute Publications
https://doi.org/10.1038/s44161-025-00740-z
Single-cell profiling reveals three endothelial-to-hematopoietic transitions with divergent isoform expression landscapes
11 November 2025
Institute Authors (6)
Robert Sellers, John Weightman, Wolfgang Breitwieser, Natalia Moncaut, Michael Lie-a-ling, Georges Lacaud
Labs & Facilities
Computational Biology Support, Molecular Biology, Genome Editing and Mouse Models
Research Group
Stem Cell Biology
11 November 2025
https://doi.org/10.1136/jitc-2025-012527
Systemic immunosuppression from ultraviolet radiation exposure inhibits cancer immunotherapy
31 October 2025
Institute Authors (4)
Isabella Mataloni, Antonia Banyard, Garry Ashton, Amaya Virós
Labs & Facilities
Mass and Flow Cytometry, Histology
Research Group
Skin Cancer & Ageing
31 October 2025
https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-24-1224/766638/Glucocorticoids-Unleash-Immune-dependent-Melanoma
Glucocorticoids Unleash Immune-dependent Melanoma Control through Inhibition of the GARP/TGF β Axis
15 October 2025
Institute Authors (12)
Charles Earnshaw, Poppy Dunn, Shih-Chieh Chiang, Maria Koufaki, Massimo Russo, Kimberley Hockenhull, Erin Richardson, Anna Pidoux, Alex Baker, Richard Reeves, Robert Sellers, Sudhakar Sahoo
Labs & Facilities
Computational Biology Support, Visualisation, Irradiation and Analysis
Research Group
Cancer Inflammation and Immunity
15 October 2025
/wp-content/uploads/2025/09/Annual_Report_2024.pdf
2024 Annual Report
23 September 2025
23 September 2025
https://doi.org/10.1182/blood.2024028033
An in vivo barcoded CRISPR-Cas9 screen identifies Ncoa4-mediated ferritinophagy as a dependence in Tet2-deficient hematopoiesis
4 September 2025
Institute Authors (1)
Justin Loke
Research Group
Myeloid Cancer Biology
4 September 2025
https://doi.org/10.1038/s41467-024-49692-1
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma
15 July 2025
Institute Authors (1)
Samra Turajlić
Research Group
Cancer Dynamics
15 July 2025
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.




















A note from the Group Leader – Santiago Zelenay
My research interest is mainly focused on understanding the mechanisms underlying natural and therapy-induced tumour immunity. In particular, the identification of the instructive signals which, by regulating the balance between tumour-promoting and tumour-suppressive inflammation, favour immune escape and therapy resistance.
To achieve our aims, I have taken significant steps towards translating my findings in the clinic, establishing a substantial local, national and international framework of collaborators, developing essential links with colleagues with expertise in immunogenomics, cancer-stroma interactions, imaging techniques, cancer biomarkers and personalised immunotherapy. I have also developed critical collaborations with expert clinical colleagues at The Christie NHS Foundation Trust as well as with colleagues in the pharmaceutical industry.