Overview
Small cell lung cancer (SCLC) comprises ~15% of lung cancers and is an aggressive neuroendocrine malignancy with poor overall survival. High circulating tumour cell (CTC) burden contributes to early, extensive and frequent oligometastatic disease.
Most patients rapidly acquire resistance to their platinum-based chemotherapy and whilst immunotherapy provides some benefit to a minority of patients, predictive biomarkers are still lacking. New, promising approaches are emerging with a recently approved DLL3-targeted therapy impacting the treatment landscape.
Beyond almost universal inactivation of TP53 and RB1, SCLC displays significant inter- and intra-tumoural heterogeneity, including a complex mutational profile and expression of lineage defining neurogenic transcription factors. Layered onto this, phenotypic plasticity contributes to disease evolution, including acquired chemoresistance.
Whilst these present significant challenges to improved outcomes for patients, greater understanding of these complex molecular features is revealing potential for personalised therapies, informed by liquid and tissue biomarkers.
Our group made a major contribution to the field of SCLC research with the development of preclinical models from patients’ CTCs termed CDX, an approach now widely used globally. We more recently discovered and characterised a rare but highly aggressive subtype of SCLC, driven by the transcription Factor ATOH1 with tropism to liver and we developed new models to study metastasis to the brain.
Featured Publications
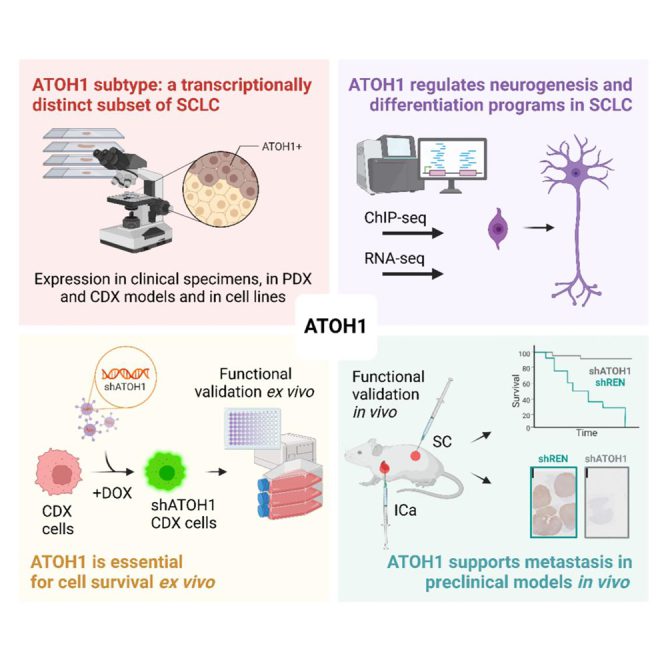
Functional characterisation of the ATOH1 molecular subtype indicates a pro-metastatic role in small cell lung cancer
27th May 2025
Catozzi et al. describe the ATOH1 SCLC molecular subtype contributing to SCLC heterogeneity. Like ASCL1 and NEUROD1, ATOH1 regulates neurogenesis. ATOH1 is associated with chemoresistance and is essential for tumor cell survival. ATOH1 supports aggressive tumor growth, including liver metastasis.
Lineage Plasticity in SCLC Generates Non-Neuroendocrine Cells Primed for Vasculogenic Mimicry
18th October 2023
Using SCLC circulating tumour cell (CTC)-derived tumour explant (CDX) models, the authors here report the first functional evidence of the role of NOTCH-driven NE to Non-NE plasticity in SCLC, facilitating vasculogenic mimicry (VM).
Soluble guanylate cyclase signalling mediates etoposide resistance in progressing small cell lung cancer
17th November 2021
Authors using paired pre-treatment and post-chemotherapy circulating tumour cell patient-derived explant (CDX) models reveal a mechanism of drug resistance in SCLC mediated by Notch and nitric oxide activation of soluble guanylate cyclase signalling.
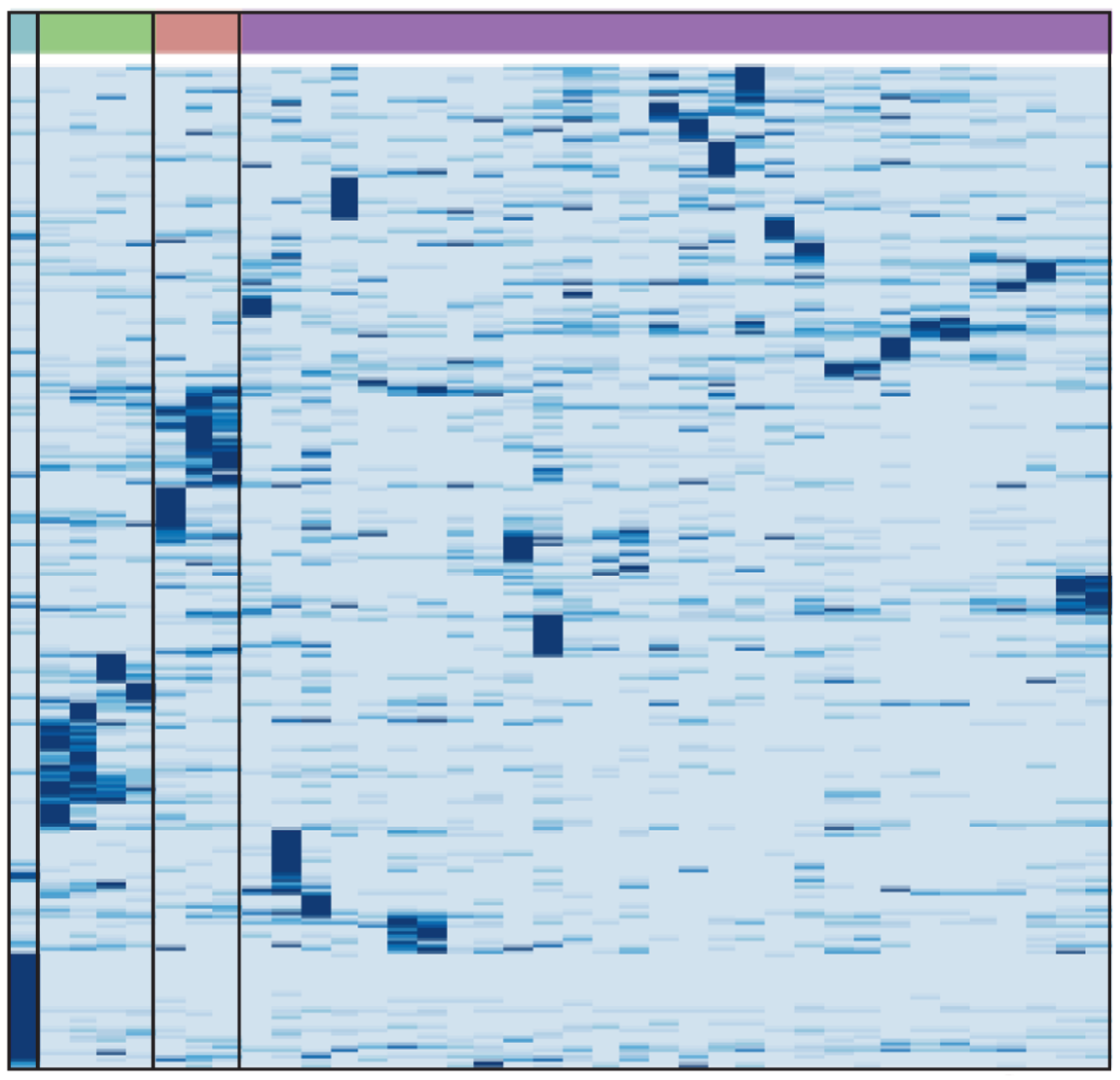
A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity
13th April 2020
Authors describe a biobank of CDX models, including CDX pairs generated pretreatment and at disease progression. The CDX biobank provides a research resource to facilitate SCLC personalised medicine.
Aims and objectives
To improve outcomes for patients with SCLC, our current goals are to uncover and exploit mechanisms of acquired chemoresistance, to better understand molecular mechanisms of metastasis to liver and to brain and to determine whether and how vasculogenic mimicry contributes to disease progression and metastasis. We are particularly interested in understanding how SCLC plasticity whereby neuroendocrine cells undergo phenotypic shift to non-neuroendocrine cells contributes to the aggressive behaviours of SCLC.
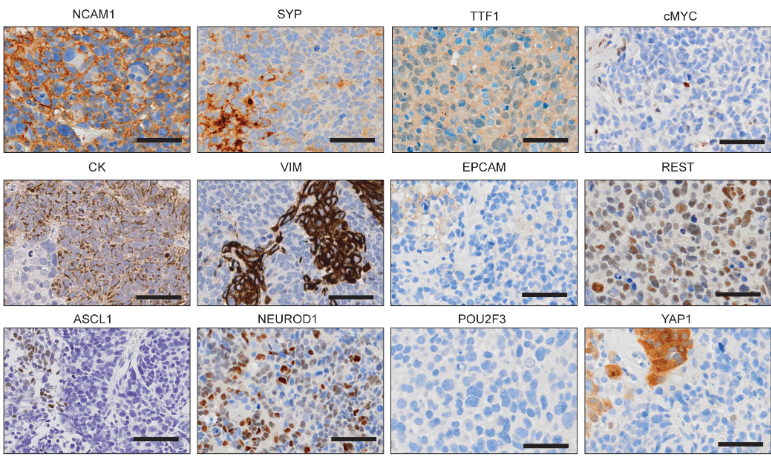
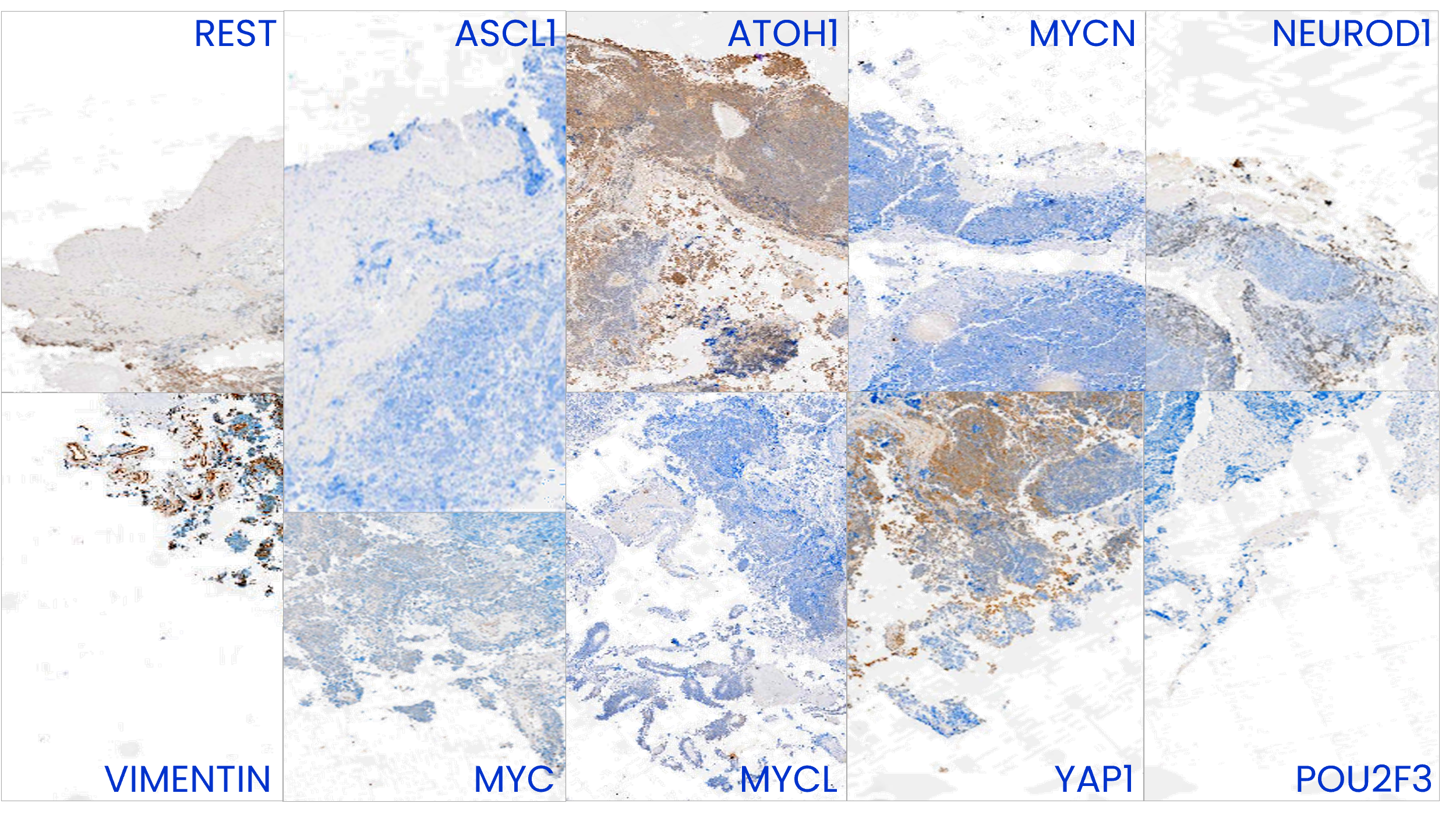
Image to the right shows phenotypic heterogeneity of SCLC reflected in IHC analysis of a CDX tumour. This tumour displays variable expression of the subtype-defining transcription factors ASCL1, NEUROD1, POU2F3 and ATOH1, different members of the MYC family (MYC, MYCL and MYCN) and other markers of SCLC plasticity and heterogeneity including YAP1, REST and Vimentin.
Meet the group
It is my great pleasure to introduce my group who are unravelling the complexity of this most recalcitrant tumour type. The team work closely with the Cancer Research UK National Biomarker Centre Preclinical Pharmacology Team, and collaborate with the Bioinformatics and Biostatistics, Nucleic Acids Biomarkers and Translational Immunology teams on all biomarker projects studying SCLC.
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.


















A note from the Group Leader – Caroline Dive
SCLC is an extraordinary cancer, a fast moving ‘shapeshifter’ that presents an enormous challenge to attaining better clinical outcomes. However, in the 15 years plus that my group has been studying this disease, clear strides forward have been made in our understanding of SCLC biology, and more recently a step forward for the minority patients who respond to immunotherapy. Despite the forward momentum in the field, there is so much more we need to understand about SCLC biology in order to find and exploit SCLC vulnerabilities. With our large biobank of patient-derived models now developed and molecularly characterised, we are beginning to comprehend new facets of SCLC heterogeneity and we remain focussed on the functional consequences of NE to non-NE plasticity, particularly as it impacts metastasis and the tumour intrinsic causes of chemoresistance. We work closely with our colleagues in the CRUK National Biomarker Centre on derivation of further models and novel therapy testing, on SCLC immunology and on liquid biopsies for clinical trials. We also benefit from the expertise across the CRUK Lung Cancer Centre of Excellence to tackle this most aggressive of lung cancers.