Overview
In our laboratory, we intend to disentangle the biological meaning of lung squamous cell carcinoma (LUSC) heterogeneity, using a multiangle approach to explore the individual and cooperative role of major LUSC drivers in the transformation of the homeostatic bronchial epithelium into preinvasive and ultimately invasive stages. Understanding the biological meaning of this LUSC evolution and inter-patient heterogeneity is necessary to design new, personalised therapeutic strategies and to gain better knowledge about the mechanisms that drive LUSC progression.
Lung squamous cell carcinoma has been historically difficult to model using genetically engineered mouse models (GEMMs), and to this date they are not sufficiently developed. In addition, LUSC has been the object of comparatively less research focus than other lung cancer types. The identification of SOX2 (frequently amplified in LUSC and a component of the squamous differentiation pathways) as the most important LUSC driver and its incorporation in LUSC modelling strategies has made LUSC models more patient relevant. However, multi-platform characterisation of large patient cohorts has revealed a complex landscape of molecular subtypes, with and without SOX2 amplification, with obscure biological origins and unknown vulnerabilities that are not represented by any existing experimental models.
Observation of the genomic landscape of LUSC has shown that unlike lung adenocarcinoma, which is dominated by alterations in the Ras pathway, there is no single targetable pathway that dominates the genomic landscape of LUSC. Instead, the most frequently altered pathways in LUSC are PI3K/Akt pathway (47%), squamous differentiation pathway (44%) and oxidative stress response (34%). Furthermore, analysis of LUSC genomes have not shown co-occurrence or mutual exclusivity in these dysregulated pathways, although alterations that dysregulate the three pathways seem to co-occur in certain molecular subtypes. This suggests that none of the pathways are indispensable in driving LUSC, but also that they can cooperate. Deciphering the biology of this complex inter-patient diversity requires individual interrogation using appropriate models to address several key questions:
- Can these pathways drive LUSC tumourigenesis per se, or there is an obligate cooperation between them?
- Are LUSC cells addicted to these pathways?
- Are these pathways mutually dependent?
- Are these pathways relevant in all LUSC molecular subtypes and if not, what processes drive LUSC in those subtypes?
In the lab, we intend to unravel the mechanisms that drive the numerous subtypes of LUSC that we observe in patients and the specific vulnerabilities of those subtypes using a new model of LUSC by genetic manipulation on human bronchial epithelial cells (HBECs).
Implementing this project requires intensive research programmes that involve the manipulation of multiple loci. Approaches to avoid a large cost in mouse lives and distress is a responsibility of the scientific community, especially in the field of LUSC, where the new availability of more relevant mouse models will increase the number of projects involving animal research. Genetic manipulation of human bronchial epithelial cells is an alternative to GEMMs, that can facilitate the modelling of LUSC heterogeneity and developmental stages. However, a proof of principle for this alternative LUSC modelling strategy is required.
Featured Publications
A human model to deconvolve genotype-phenotype causations in lung squamous cell carcinoma
4th April 2025
Authors describe a unique model of lung squamous cell carcinoma (LUSC) using genetically modified primary human bronchial basal cells. This strategy constitutes an alternative and patient-relevant system to model LUSC and identify genotype-phenotype correlations relevant for LUSC medicine and early detection.
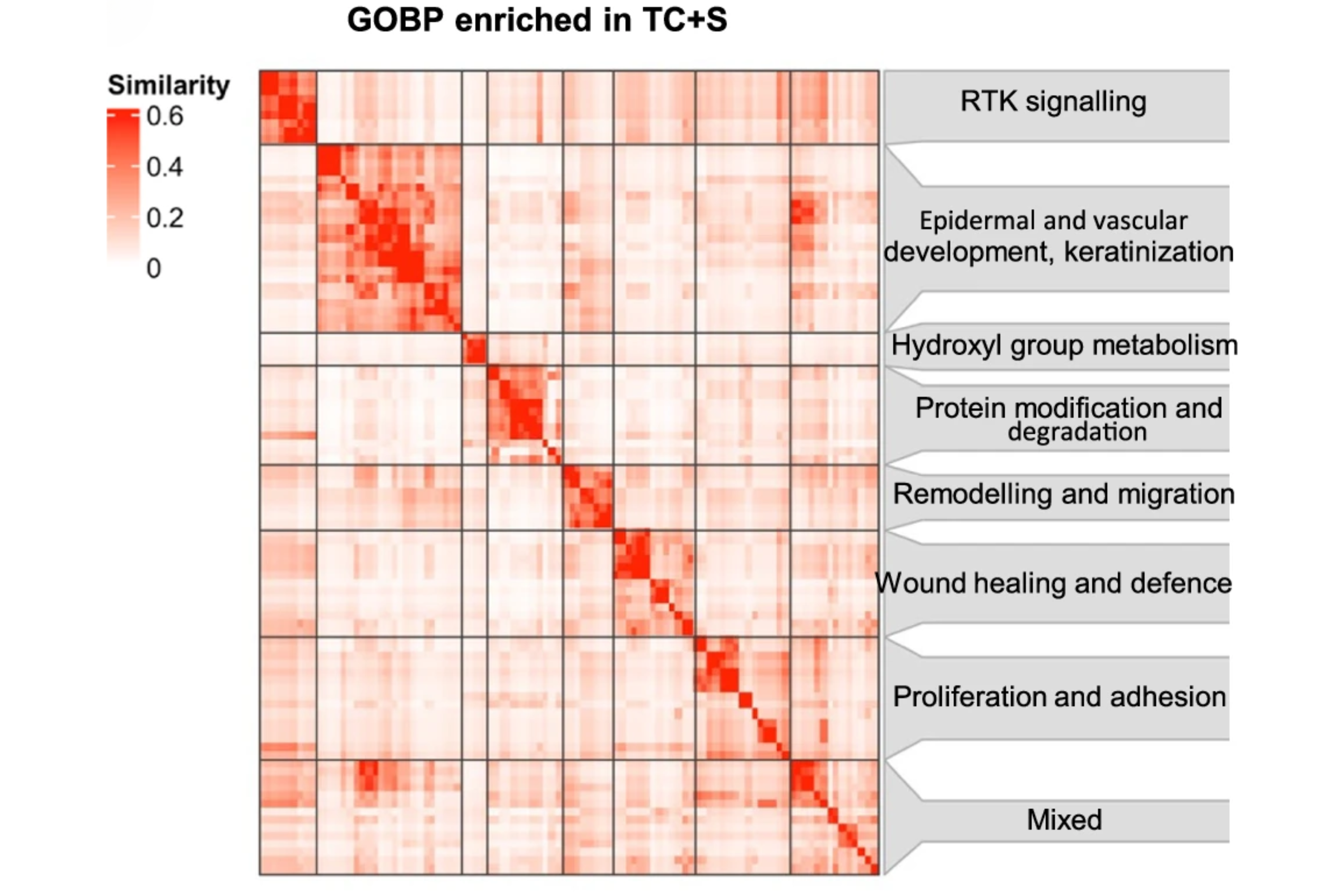
Interrogating the precancerous evolution of pathway dysfunction in lung squamous cell carcinoma using XTABLE
9th March 2023
The authors have created a resource tool that is valuable in assessing precancerous lesions in the lung, which may serve as a tool for investigators working in this area, and as an example for additional similar resources. The accessibility of the tool is a concern but does not diminish the quality of the product.
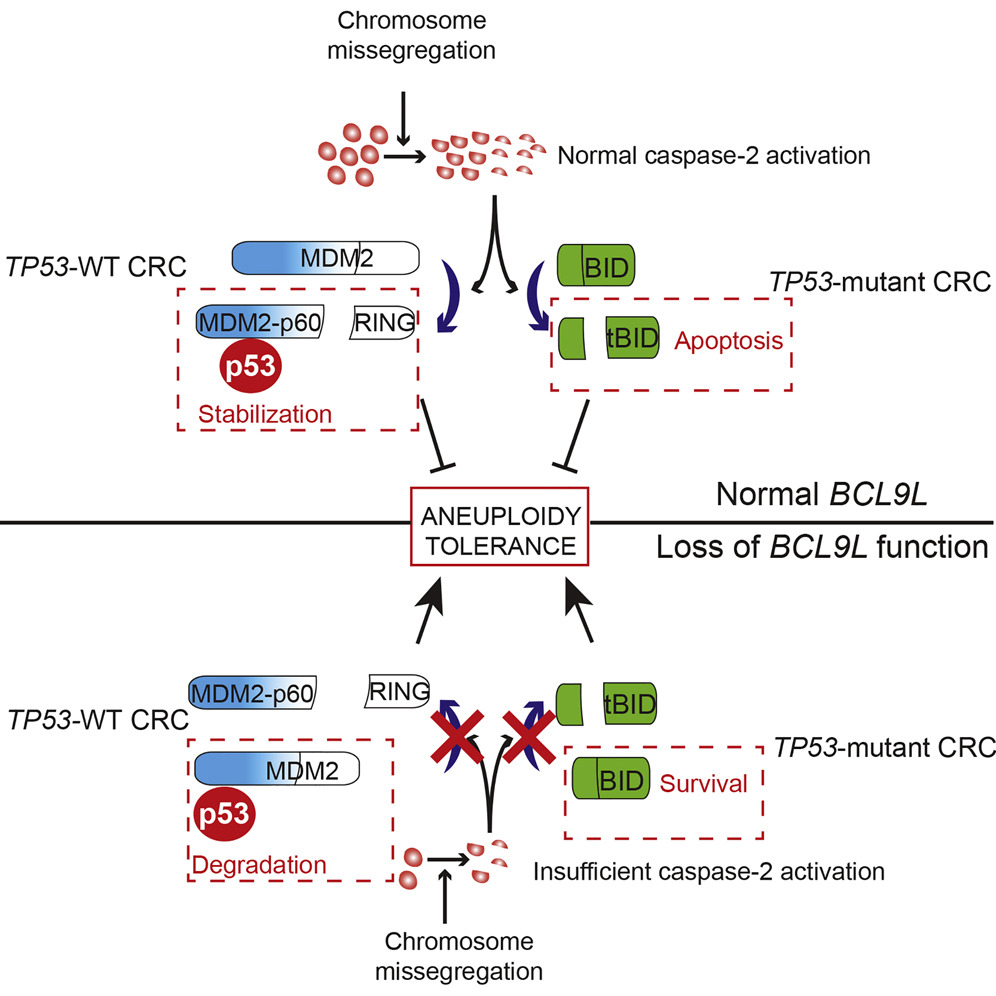
BCL9L Dysfunction Impairs Caspase-2 Expression Permitting Aneuploidy Tolerance in Colorectal Cancer
9th January 2017
Lopez-Garcıa et al. find that BCL9L is often genetically inactivated in human colorectal cancers with chromosomal instability. BCL9L dysfunction promotes aneuploidy tolerance by reducing basal caspase-2 levels and preventing cleavage of MDM2 and BID independent of TP53 mutation status.
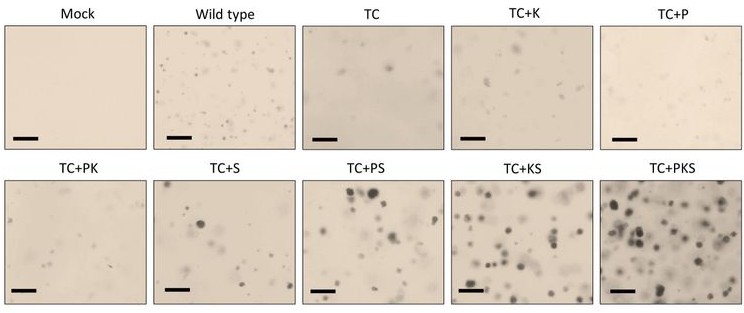
A novel human model to deconvolve cell-intrinsic phenotypes of genetically dysregulated pathways in lung squamous cell carcinoma
14th December 2023
Authors report an alternative and unique in vitro model of lung squamous cell carcinoma (LUSC) using primary human bronchial epithelial cells (hBECs) from three healthy donors.
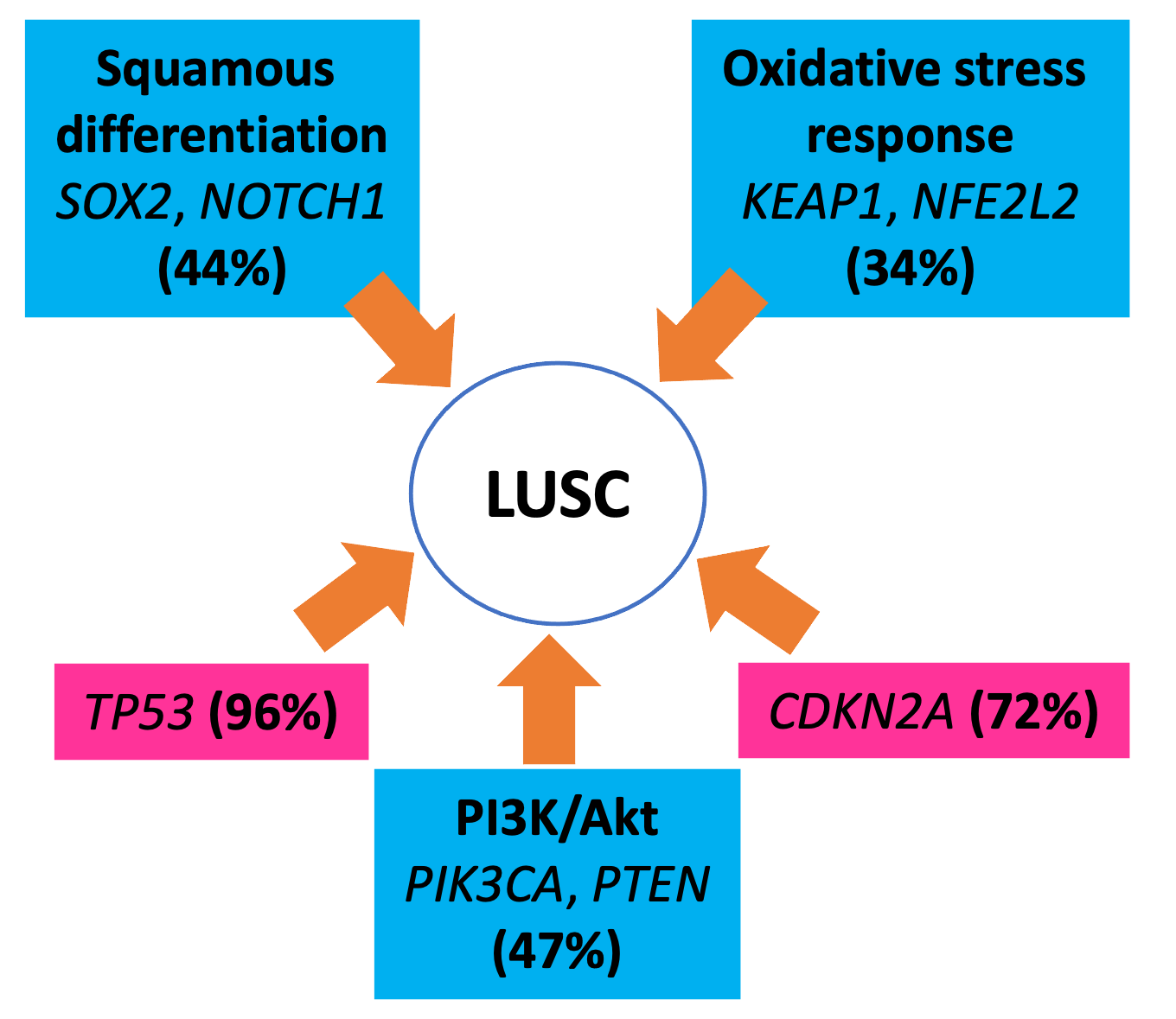
Genomic landscape of LUSC
Observation of the genomic landscape of LUSC has shown that unlike lung adenocarcinoma, which is dominated by alterations in the Ras pathway, there is no single targetable pathway that dominates the genomic landscape of LUSC. Instead, the most frequently altered pathways in LUSC are PI3K/Akt pathway (47%), squamous differentiation pathway (44%) and oxidative stress response (34%).
This figure shows summary of the most relevant tumour suppressors (pink boxes) and pathways (blue boxes) involved in LUSC development, with examples of pathway components altered in LUSC and the percentage of cases with at least one alteration targeting the pathway.
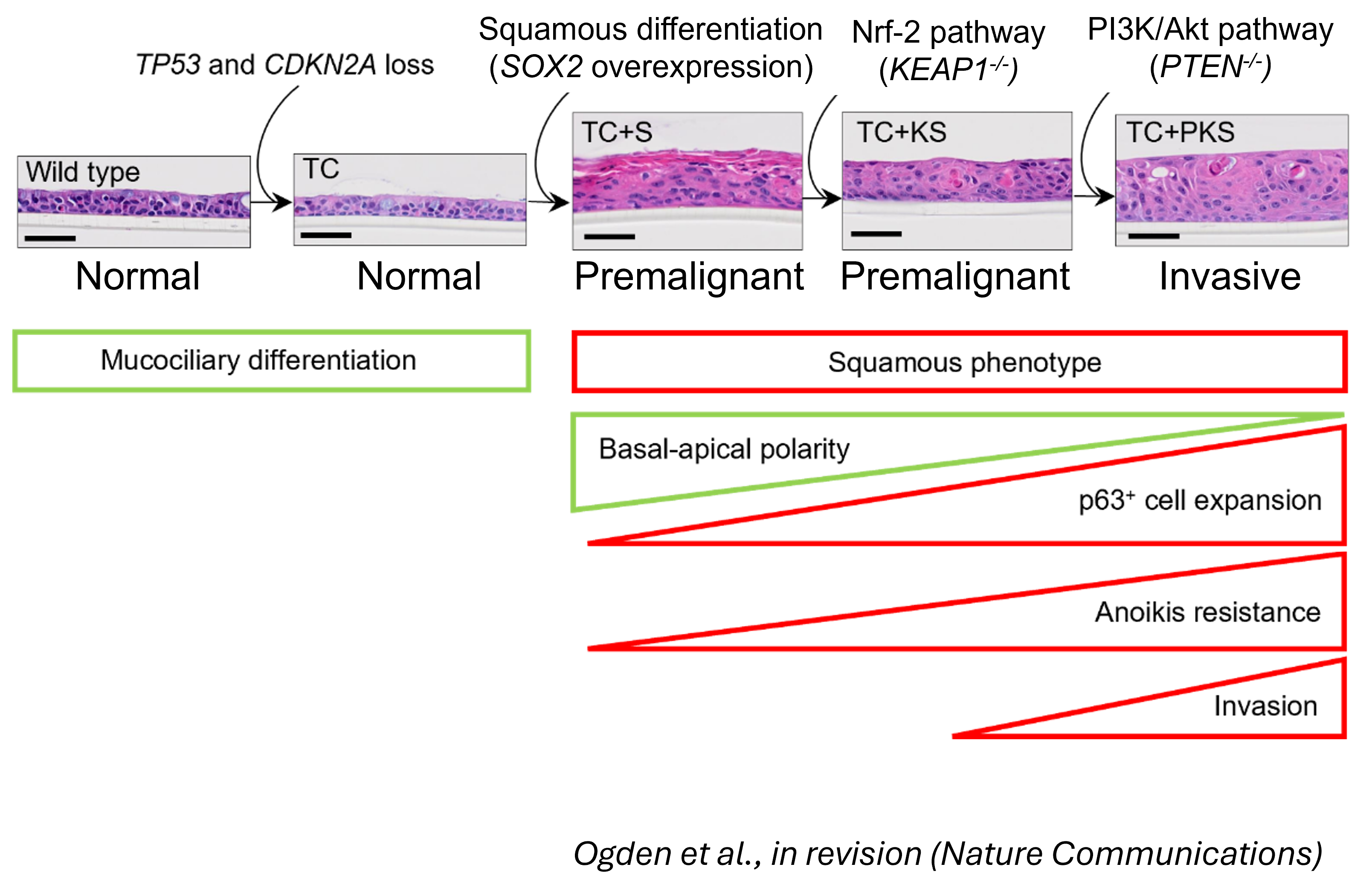
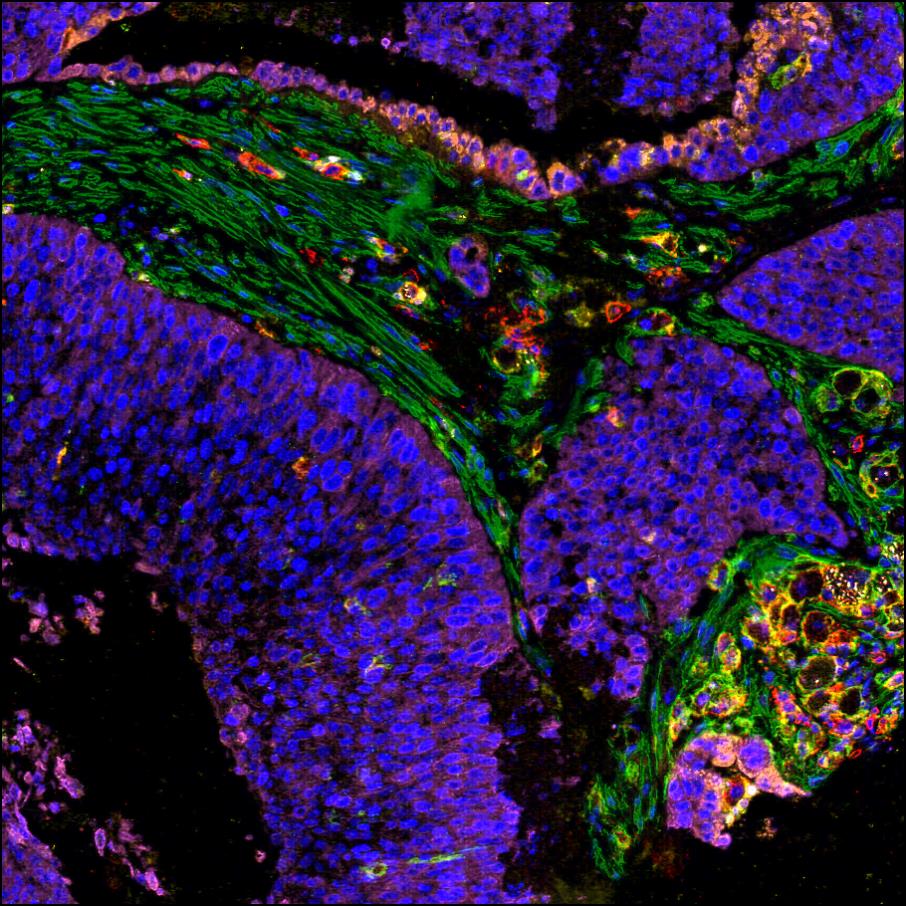
New model of LUSC using HBECs
The group intend to unravel the mechanisms that drive the numerous subtypes of LUSC that we observe in patients and the specific vulnerabilities of those subtypes using a new model of LUSC by genetic manipulation on human bronchial epithelial cells (HBECs).
Current methodologies permit efficient expansion of HBECs, genome editing and development of organoids mimicking bronchial morphology. Using these methodologies, we can disentangle how driver alterations induce epithelial perturbations indicative of LUSC initiation and progression. Additionally, HBECs reflect human diversity better than mouse models, constitute a more adequate system to investigate the effect of exposures, mainly smoking, and predisposition.
Figure shows haematoxylin-eosin stained sections or air-liquid interface (ALI) HBEC cultures from wild-type and the mutants HBECs that follow the most likely evolutionary trajectory of LUSC inferred from our results. The diagram below shows the phenotypic changes associated with this evolutionary trajectory.
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.












A note from the Group Leader – Carlos Lopez-Garcia
I am mainly an epithelial biologist interested in understanding what mechanisms determine the transition from a normal epithelium into invasive carcinomas by deconvolving how frequently dysregulated pathways cooperate to alter epithelial homeostasis. I am also interested in finding alternatives to mouse models with two objectives: reducing the use of mice in cancer research and developing models that recapitulate better the human biology.
I am now focusing on lung squamous cell carcinoma to develop my research program. However, I intend to extend my research to other squamous carcinomas, as they are a group of diseases with similar biological features that are likely to show common vulnerabilities.