Overview
The goal of the Leukaemia Biology group is to develop understanding of disease mechanisms in myeloid lineage blood cancers and through doing so to identify candidate therapeutic targets for development through to the clinic.
Recent years have seen significant progress in the development of better therapies for people with blood cancer, with concomitant improvements in response. However, there remains a substantial unmet need for more effective and less toxic treatments.
Much of the focus of the group continues to be on understanding disease mechanisms in acute myeloid leukaemia (AML). This is a blood cancer characterised by a block to normal myeloid lineage differentiation leading to accumulation of myeloid blast cells in bone marrow (BM), with failure of normal blood cell production. Despite much progress in recent years, including the FDA approval of several novel therapies, it remains the case that long term survival from AML remains poor, especially in those over the age of 60.
The group works on understanding how transcription factors and their associated chromatin cofactors sustain myeloid blood cancers such as AML. We recently reported our discovery of how a small molecule bromodomain inhibitor of the acetyltransferases EP300 and CBP induces cell cycle arrest and cellular differentiation in blood cancer, as well as our preliminary data from the early phase clinical trial evaluation of CCS1477, where we see promising signs of clinical activity across a range of haematological malignancies.
Featured Publications
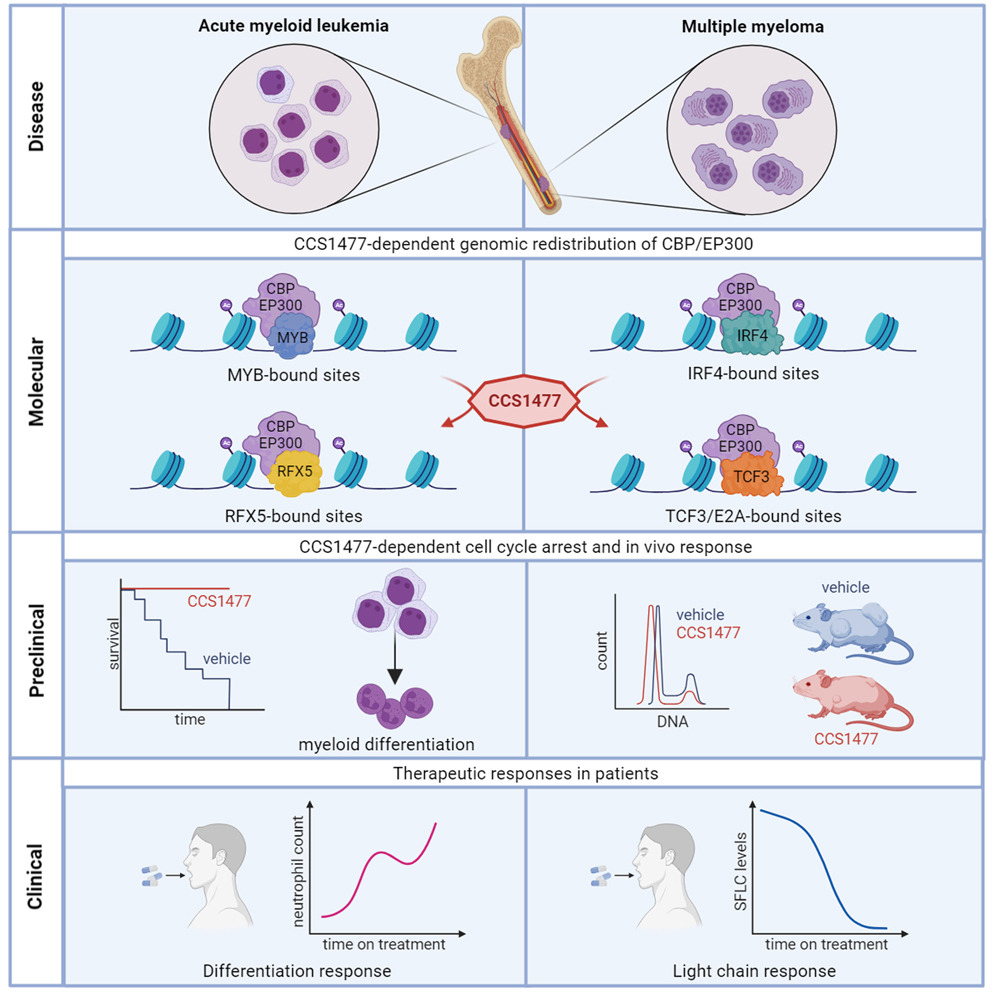
Therapeutic targeting of EP300/CBP bybromodomain inhibition in hematologicmalignancies.
22nd November 2023
EP300/CBP are histone acetyltransferases recruited onto chromatin by oncogenic transcription factors and control the transcriptional program via their activity in enhancer areas. Nicosia et al. offer new promise in targeting EP300/CBP using the small-molecule inhibitor CSS1477 in patients with blood tumours and no other therapeutic options.
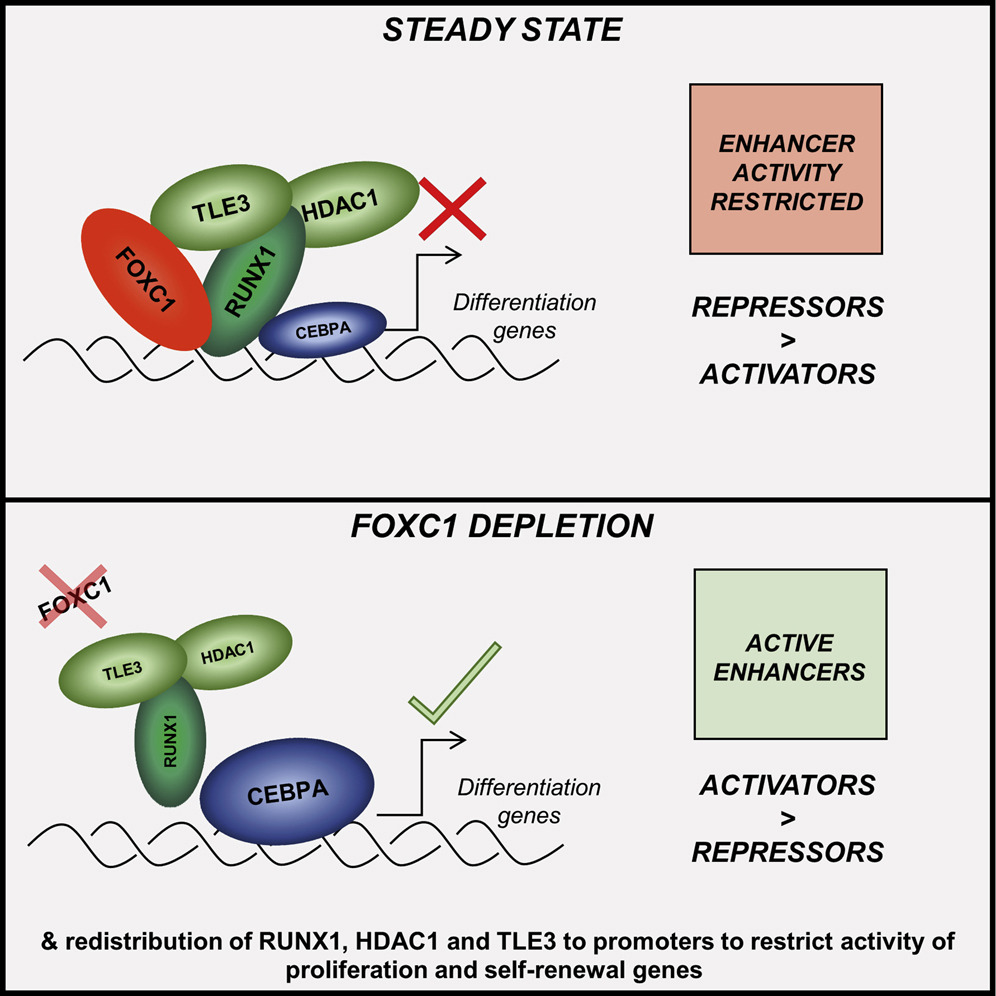
Enhancer recruitment of transcription repressors RUNX1 and TLE3 by mis-expressed FOXC1 blocks differentiation in acute myeloid leukemia
21st September 2021
• FOXC1 contributes to a monocyte-macrophage lineage differentiation block in AML • FOXC1 and RUNX1 colocalize on chromatin via interaction of their DNA binding domains • A FOXC1/RUNX1/TLE3 repressor complex limits monocyte gene enhancer activity • FOXC1 depletion in AML initiates widespread redistribution of RUNX1/TLE3 on chromatin
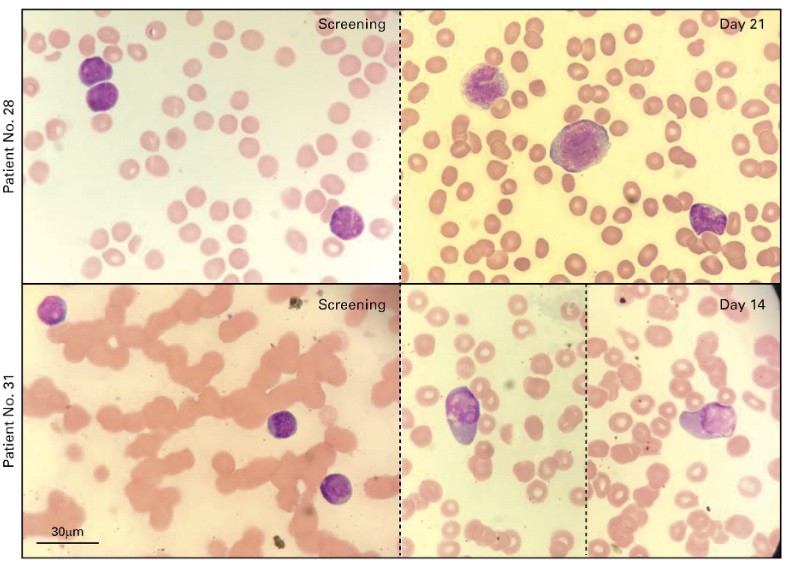
First-in-Human Phase I Study of Iadademstat (ORY-1001): A First-in-Class Lysine-Specific Histone Demethylase 1A Inhibitor, in Relapsed or Refractory Acute Myeloid Leukemia
14th October 2020
Morphologic response to treatment with iadademstat. (A) Representative images of blood smears showing morphologic differentiation from patient 28 (top) at screening (left) and cycle 1(C1), day 21 (D21) (right) and patient 31 (bottom) at screening (left) and C1D14 (right; two images from the same slide and patient are shown, separated by a dotted line).
Meet the group
Here are the members of my lab. They are a great bunch of hard working and accomplished scientists and it is a pleasure to work with them.

Senior Group Leader

Senior Scientific Officer

Postdoctoral Scientist
All Institute Publications
https://doi.org/10.1038/s44161-025-00740-z
Single-cell profiling reveals three endothelial-to-hematopoietic transitions with divergent isoform expression landscapes
11 November 2025
Institute Authors (6)
Robert Sellers, John Weightman, Wolfgang Breitwieser, Natalia Moncaut, Michael Lie-a-ling, Georges Lacaud
Labs & Facilities
Computational Biology Support, Molecular Biology, Genome Editing and Mouse Models
Research Group
Stem Cell Biology
11 November 2025
https://doi.org/10.1136/jitc-2025-012527
Systemic immunosuppression from ultraviolet radiation exposure inhibits cancer immunotherapy
31 October 2025
Institute Authors (4)
Isabella Mataloni, Antonia Banyard, Garry Ashton, Amaya Virós
Labs & Facilities
Mass and Flow Cytometry, Histology
Research Group
Skin Cancer & Ageing
31 October 2025
https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-24-1224/766638/Glucocorticoids-Unleash-Immune-dependent-Melanoma
Glucocorticoids Unleash Immune-dependent Melanoma Control through Inhibition of the GARP/TGF β Axis
15 October 2025
Institute Authors (12)
Charles Earnshaw, Poppy Dunn, Shih-Chieh Chiang, Maria Koufaki, Massimo Russo, Kimberley Hockenhull, Erin Richardson, Anna Pidoux, Alex Baker, Richard Reeves, Robert Sellers, Sudhakar Sahoo
Labs & Facilities
Computational Biology Support, Visualisation, Irradiation and Analysis
Research Group
Cancer Inflammation and Immunity
15 October 2025
/wp-content/uploads/2025/09/Annual_Report_2024.pdf
2024 Annual Report
23 September 2025
23 September 2025
https://doi.org/10.1182/blood.2024028033
An in vivo barcoded CRISPR-Cas9 screen identifies Ncoa4-mediated ferritinophagy as a dependence in Tet2-deficient hematopoiesis
4 September 2025
Institute Authors (1)
Justin Loke
Research Group
Myeloid Cancer Biology
4 September 2025
https://doi.org/10.1038/s41467-024-49692-1
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma
15 July 2025
Institute Authors (1)
Samra Turajlić
Research Group
Cancer Dynamics
15 July 2025
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.


















A note from the Group Leader – Tim Somervaille
Recent years have seen significant progress in the development of better therapies for people with blood cancer, with concomitant improvements in response. However, there remains a substantial unmet need for more effective, and less toxic, treatments. For example, outcomes in acute myeloid leukaemia (AML) are particularly poor in older adults and those with relapsed or refractory disease, and malignancies such as multiple myeloma are incurable for the great majority. The overarching goal of our group at the Cancer Research UK Manchester Institute is to deliver a bench-to-bedside programme of blood cancer research. Much of our effort is focussed on understanding how transcription factors and their associated chromatin cofactors sustain myeloid blood cancers such as AML. In keeping with this, in recent years we have worked with colleagues in pharma and in the clinic to bring forward novel therapies targeting the histone demethylase LSD1, and the bromodomain acetyltransferases EP300 and CBP.