Overview
Approximately 80% of patients with SCLC are diagnosed with metastases. A major barrier to understanding this metastasis has been lack of robust and reliable metastatic preclinical and/or patient-derived models, combined with a paucity of biopsies from metastatic sites, often only obtained at autopsy. Pertinent to this project, using our in vivo resection protocol, multiple subcutaneously implanted then resected CDX tumours routinely, spontaneously metastasise to multiple organs with similar tropism of patients, including to the liver and the brain. Intra-tumoural plasticity whereby NE cells (regardless of TF subtype) undergo a NOTCH-driven NE to non-NE phenotype transition is also recapitulated in short term cultures of several CDX models (separable by nonNE adherence to plastic). Earlier studies in a SCLC Genetically Engineered Mouse Model suggest that NE and nonNE cell-cooperation is needed for metastasis. However, how this co-operation underpins metastasis remains unclear.
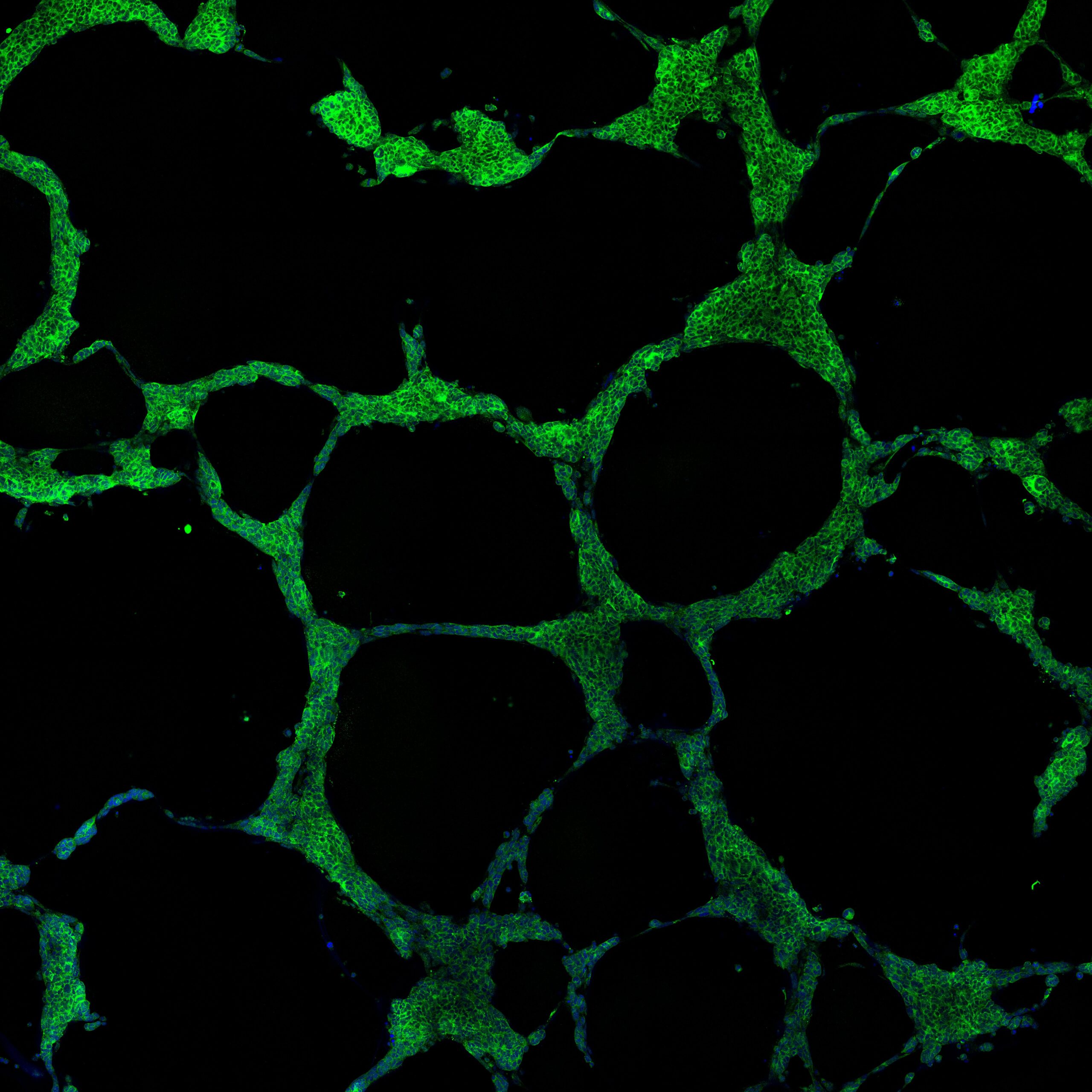
We recently reported the first example of functional SCLC intra-tumoral heterogeneity, whereby NE cells that have undergone the nonNE phenotype transition can undergo Vasculogenic Mimicry (VM). This is a form of cellular plasticity where tumour cells trans-differentiate to acquire endothelial-like properties and form de novo vessels independently of angiogenesis. VM is observed in most of our CDX models where VM vessels co-localise with the non-NE marker REST in vivo. Ex vivo cultures on Matrigel of isolated non-NE and nonNE cell populations from the same CDX tumour showed that only non-NE cells, not NE cells, formed hollow tubules that we and others consider a surrogate for VM competency. Tubule formation required NOTCH signalling. In vivo studies revealed that VM vessels are perfused. Hypoxia is a known driver of cancer cell plasticity, survival, drug resistance and metastasis.
Whilst hypoxia is implicated as a microenvironmental cue for VM in multiple tumour types and we have some evidence for this in SCLC CDX, the precise mechanism of hypoxia driven VM is unclear. Ex vivo cultures of non-NE exhibit pseudohypoxic traits with elevated hypoxia inducible factor 1 (HIF1) levels. Large CDX tumours showed an inverse correlation between hypoxia and number of VM vessels suggesting VM has been activated to overcome limited O2 supply. Our hypothesis is that nonNE cell VM stimulated by hypoxia allows tumours rapid access to oxygen and nutrients to support continued growth. HIF proteins can also be stabilised under non-hypoxic conditions via oncogenes such as MYC, which is upregulated in SCLC non-NE cells. In this project we seek to unravel the associations between hypoxia, pseudo hypoxia, VM and metastasis.
Image: CDX models can be cultured ex vivo to enable phenotypic characterisation of SCLC heterogeneity and plasticity. Non-Neuroendocrine CDX cells cultured on Matrigel form tubules indicative of Vasculogenic Mimicry competence and can be visualised with an immunofluorescence antibody against human mitochondria (green).