Skin Cancer & Ageing team show how lipids influence metastasis and tropism
Institute Fellow Amaya Viros and her team work on why some melanomas are more metastatic. Recently they have focused on how ageing influences melanoma metastasis to solid organs, with implications for therapy response – work that has now been published in Cancer Cell.
Cutaneous melanoma can metastasise to distant sites, and this spread to organs is the key driver of death.
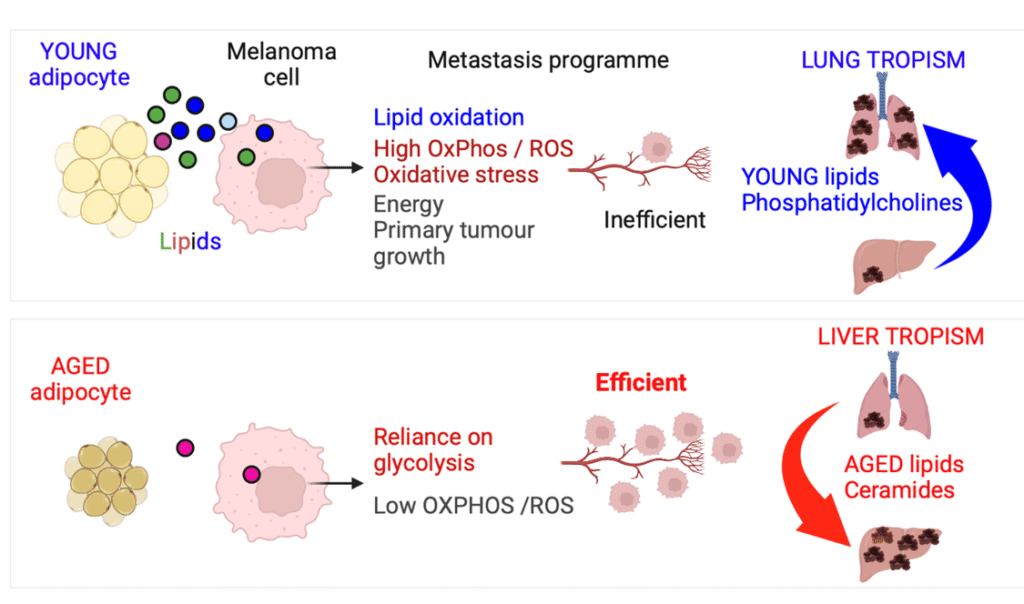
Our work shows that lipids in the skin decrease with age. Lipids available in the skin are nutrients that can be taken up by melanoma cells to fuel tumour growth and impact tumour behaviour.
We found that lipids in young skin are very abundant and although they promote local tumour growth, they do not promote melanoma metastasis.
By contrast, lipids in aged skin are less abundant, but some aged-specific lipid species taken up by melanoma cells drive early metastasis, predominantly to the liver.
Amaya Viros
CRUK Advanced Clinician Scientist | Skin Cancer & Ageing group
The team are uncovering why melanoma is more metastatic in older people, why it targets visceral organs, and how therapy responses differ between younger and older people.
Following the observation that liver metastases are more common in older people, while in younger people brain metastases are more frequent, they investigated so called age-related factors that instruct melanoma cells to colonise specific organs.
Tumour cells undergo metabolic changes when they metastasise so they can adapt to the changing microenvironment. Until now, the tumour microenvironment cues that impact metastasis and tropism were unknown.
In this paper, the researchers show how subcutaneous lipids, which support normal skin function, impact melanoma metastasis and tropism – the distribution of cancer cells to specific organs.
Brightfield image of differentiated mouse adipocytes. Differentiation is marked by the development of intracellular lipids (lipid bubbles inside the cell) upon administration of differentiation media.
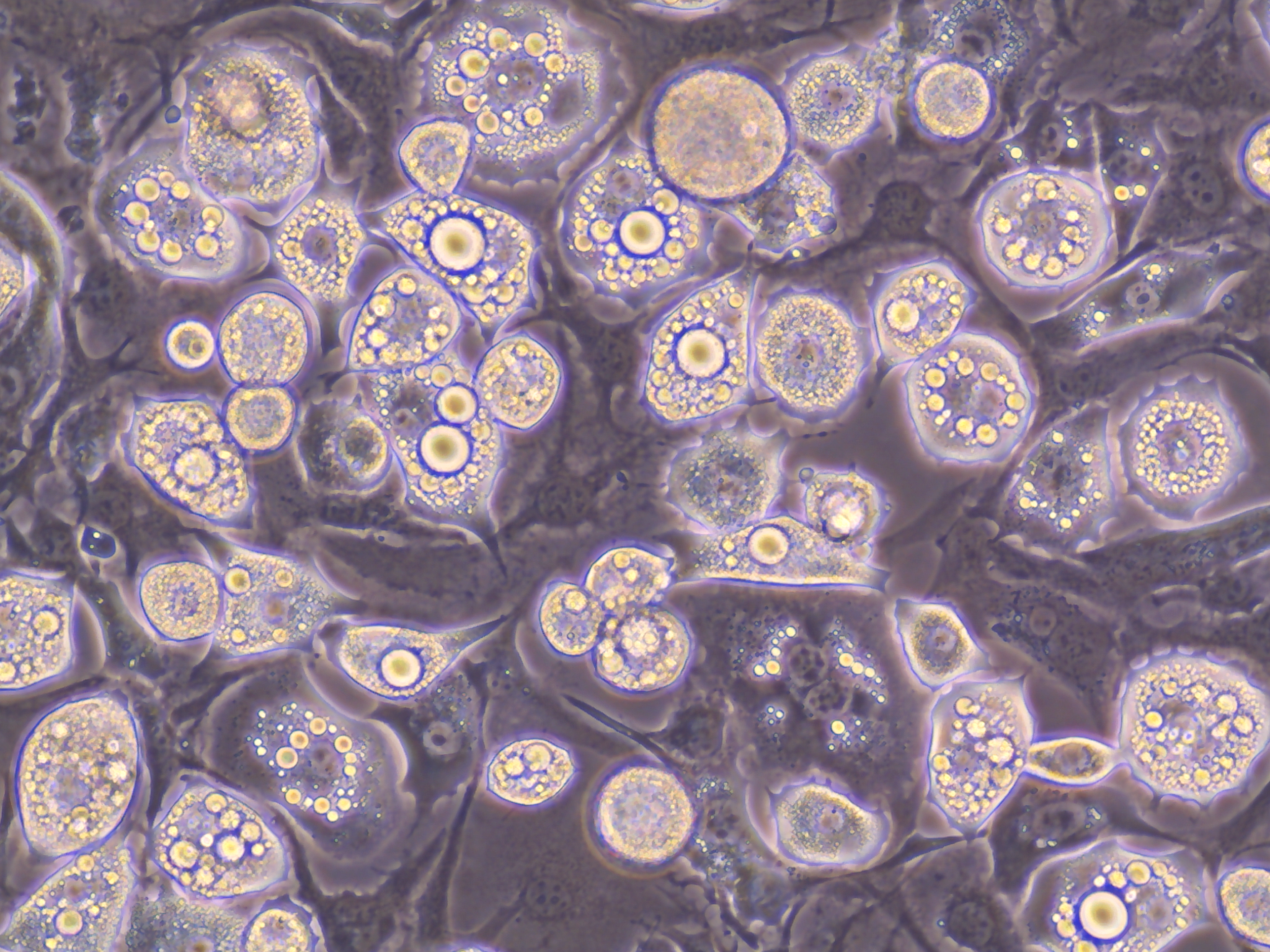
Shilpa Gurung et al show that as we age, there is a total loss in the number of adipocytes in the skin, along with a loss of lipid production and secretion.
They find in young skin – where lipid availability is higher – melanoma cells oxidise lipids as an energy source, leading to oxidative phosphorylation (OXPHOS) and oxidative stress, which in turn limits the metastasis of cells in blood.
In contrast, older skin has fewer lipids, so melanoma cells do not rely on lipid oxidation for energy. Consequently, they have lower OXPHOS and oxidative stress and are highly metastatic. Lower OXPHOS, imposed by lipid cues in the cutaneous microenvironment, drives liver metastasis.
Furthermore, lipids taken up by melanoma cells are signalling molecules that drive cancer pathways.
Our findings show that targeting lipid availability in the melanoma tumour microenvironment limits metastatic spread to the brain, lung and liver.
The lipids that need to be targeted differ depending on the age of the host.
Amaya Viros
CRUK Advanced Clinician Scientist | Skin Cancer & Ageing group
Simply put, the Skin Cancer & Ageing team found that melanoma cells co-opt lipids from the skin, which vary in availability by age, and use them as nutrients to fuel growth, where metabolic adaptation to nutrient availability dictates metastasis and tropism.
These findings have important implications for therapy, at a time when the UK population is ageing rapidly. This paper identifies different, age-specific therapeutic targets that could delay melanoma spread in people across different age groups.




