New model could be missing piece of the puzzle scientists need to advance research into the treatment of lung cancer
Patients with lung cancer have poor survival outcomes, and those treated successfully sometimes face long-term side effects.
Identifying the driving force behind the formation of Lung Squamous Cell Carcinoma is a key step to improving survival. Research like this opens the door to better visualise the efficacy of drug treatments on cancer cells
Iain Foulkes
Executive Director of Research and Innovation, Cancer Research UK
Scientists at the Cancer Research Manchester Institute have created a new model of Lung Squamous Cell Carcinoma (LUSC), a common type of lung cancer, to study the development, activity, and vulnerabilities of the disease.
In the UK, around 80 to 85% of lung cancers are non-small cell lung cancer, of which squamous cell carcinoma is one of the main types. Almost one in three lung cancer patients have LUSC. Despite this, the disease remains poorly understood and treatment options are limited. Just one in five (21%) of lung cancer patients in England survive their disease for five years or more.
Researchers say the new cell-based model could be studied and tested on to develop more effective treatments. It also has the potential to improve early detection of LUSC as it accurately reproduces the early stages of the disease.
The study, led by Carlos Lopez-Garcia, was published in Nature Communications and was funded by Cancer Research UK and the NC3Rs, a UK-based scientific organisation that aims to replace, reduce and refine the use of animals in research, along with support from the Rosetrees Trust.
With this new strategy to model lung squamous cell carcinoma, we are in a much better position to understand how this disease progresses. Developments like these accelerate research on new therapies and improve existing ones, powering us through clinical advancements for years to come.
Carlos Lopez-Garcia
Institute Fellow, CRUK Manchester Institute
New models can also help to bridge the gap between animal testing and clinical trials, a gap which makes it harder to take drugs from the lab to the bedside. While mouse models play a critical role in cancer research, tests are not always reproducible in humans. Importantly, this new LUSC model will both offer an alternative to animal testing and allow faster, cheaper, and larger experiments to be conducted.
To build their comprehensive LUSC model, scientists took healthy lung cells from three donors and used sophisticated techniques, including CRISPR gene editing, to make the cells cancerous. This step-by-step process enabled the team to unravel the mutations needed to turn healthy cells into cancer cells, which was replicable across all three donors.
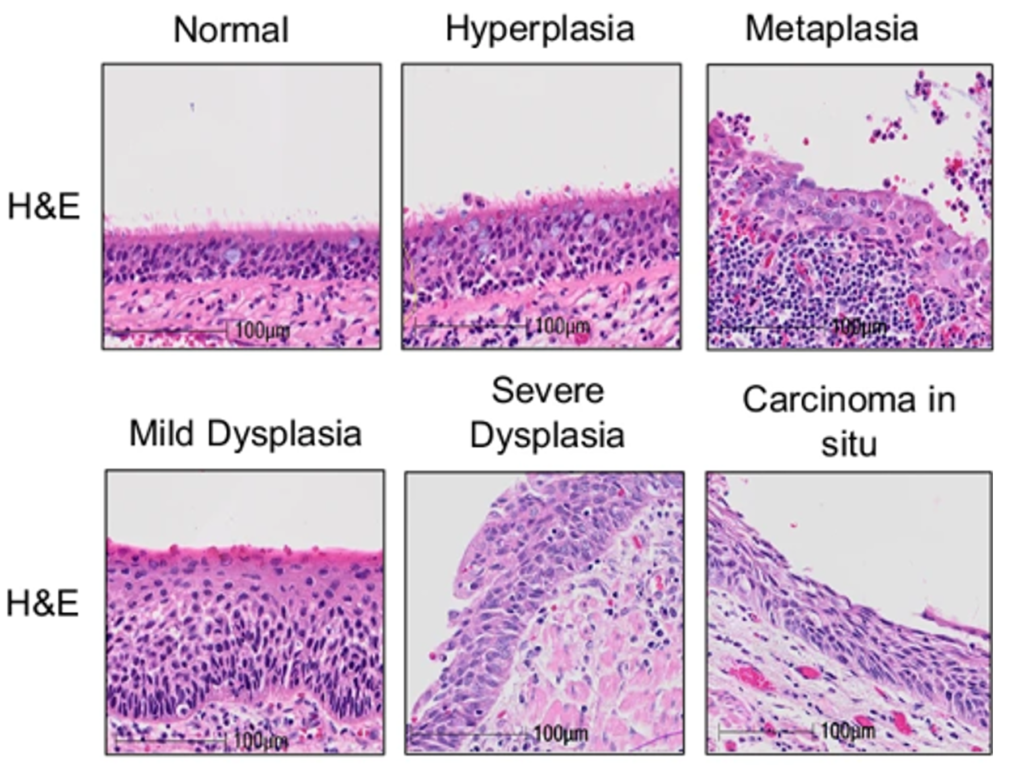
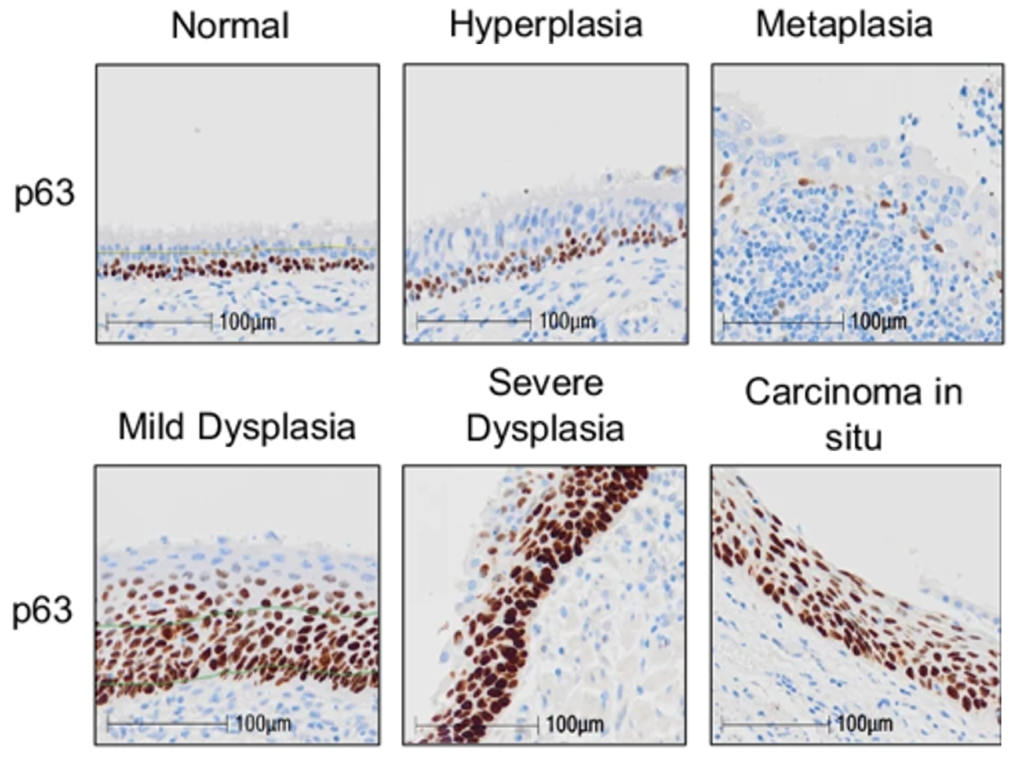
These new models are helping us gain a better understanding of how lung cancer develops and offer a very exciting opportunity to find new therapeutic vulnerabilities in this hard-to-treat cancer. It is also exciting to see an approach like this which helps bridge the gap between research in the lab and in animal models, which ultimately brings benefit to patients.
Caroline Dive
Interim Director of the Cancer Research UK Manchester Institute, and Director of the CRUK National Biomarker Centre




