Overview
Cancer metastasis is deeply influenced by the host’s biology. Cancer interacts dynamically with the host’s immune system, metabolism, and microenvironment, including surrounding tissues, blood vessels, and extracellular components. Host factors such as age, sex, diet, genetics and comorbidities shape tumour progression, response to therapy, and metastatic patterns. We aim to understand how host-specific factors and site-specific cues drive melanoma progression and therapeutic outcomes. Our aim is to develop personalised melanoma rationales that consider the entire organism, not just the tumour itself.
Our group aims to understand the melanoma metastatic process to solid organs, and how melanoma adapts to new environments and how new sites impact the response to therapy. Our aim is to find vulnerabilities during the metastatic process that we can target and change patient outcomes, reducing relapses.
We are studying the transcriptional and metabolic programmes that affect the melanoma metastatic process, from the primary site (skin) to solid organs. We integrate next generation sequencing, mass cytometry, metabolic profiling and imaging of cancer cells and the tumour microenvironment to profile how metastases develop in different hosts and organs.
We use mouse and human tissue to study spatial localisation to study the physical association between stroma and cancer cells. We aim to elucidate the programmes in primary tumours that promote metastasis, the similarities between primary and metastatic sites, as well as the site-specific differences that are linked to progression and therapy resistance.
Finally, we are developing biomarkers to predicts metastasis from primary sites.
Featured Publications

Systemic immunosuppression from ultraviolet radiation exposure inhibits cancer immunotherapy
31st October 2025
Authors show systemic UVR exposure increases circulating regulatory T cells and induces an immunosuppressive signature within colorectal and melanoma mouse tumors, thereby impairing the efficacy of immunotherapy.
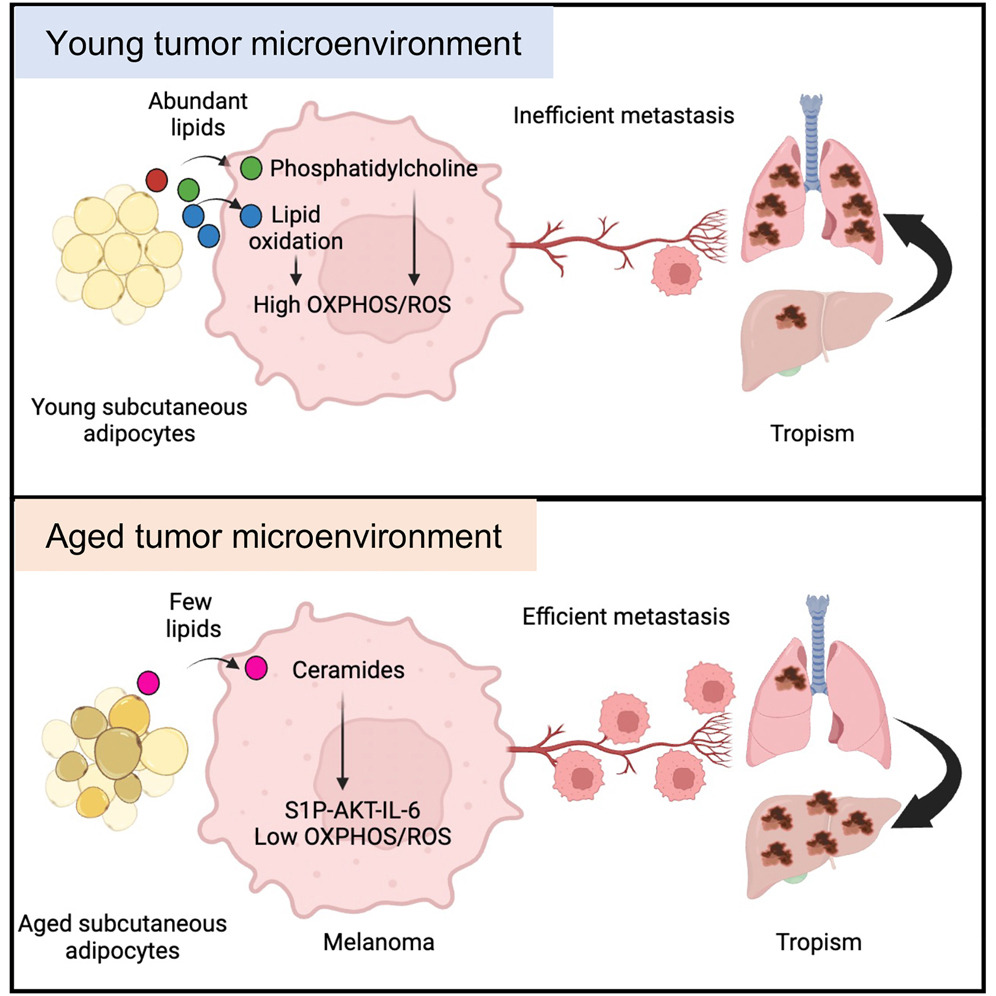
Stromal lipid species dictate melanoma metastasis and tropism
24th April 2025
Gurung et al. show that young subcutaneous adipocytes provide lipids and phosphatidylcholine to melanoma cells, which activates PI3K-AKT, OXPHOS, and oxidative stress. High OXPHOS reduces metastatic burden and associates with lung tropism. Conversely, aged subcutaneous adipocytes provide ceramides to melanoma cells, which activates S1P-STAT3-IL-6 signaling, increasing total metastatic burden and liver metastasis.

Ultraviolet light-induced collagen degradation inhibits melanoma invasion
12th May 2021
Authors studied how UVR modifies dermal fibroblast function, the extracellular matrix (ECM) and melanoma invasion. They highlight the prognostic power of collagen in aged primary melanoma tumours.

Female Immunity Protects from Cutaneous Squamous Cell Carcinoma
1st June 2021
Sex bias affects cancer incidence, mortality, and therapy response; and the molecular landscape of cancer differs by sex. This work reveals men are more susceptible to cutaneous aggressive squamous cell carcinoma, in contrast to women who activate stronger immune responses when challenged with the same carcinogens.

Molecular subtype, biological sex and age shape melanoma tumour evolution
1st February 2021
The authors use mathematical modelling of somatic mutations in 396 melanomas from The Cancer Genome Atlas to evaluate how sex and age collectively influence TMB, subtype and mutational signatures and confirmed that clock‐like mutations accumulate with age.

Molecular characterization of fast-growing melanomas
1st February 2022
Fast-growing melanomas are aggressive and linked to early death. Rapid growth is more frequent in patients with less accumulated sun exposure and is associated with thicker, ulcerated tumors with fibroblast growth factor receptor 2 mutations. Ulceration, thickness, and fibroblast growth factor receptor 2 mutations are biomarkers for aggressive disease and could stratify patients for adjuvant therapy.
Meet the group
It is a pleasure to introduce my team who work to deliver our research goals. We work in a friendly and collaborative environment, supporting each other’s projects.

Institute Fellow
FAQs
Yes, I continue to see NHS patients in my weekly general dermatology and high-risk skin cancer clinic at Salford Royal NHS. It is a very important part of our work, as it is always patient concerns that guide our next scientific experiments.
We are keen to expand our research network and work collaboratively within the University of Manchester, in the UK and internationally. Get in touch with us using the contact form below, we’re always open to new ideas and collaborations.
All Institute Publications
https://doi.org/10.1038/s44161-025-00740-z
Single-cell profiling reveals three endothelial-to-hematopoietic transitions with divergent isoform expression landscapes
11 November 2025
Institute Authors (6)
Robert Sellers, John Weightman, Wolfgang Breitwieser, Natalia Moncaut, Michael Lie-a-ling, Georges Lacaud
Labs & Facilities
Computational Biology Support, Molecular Biology, Genome Editing and Mouse Models
Research Group
Stem Cell Biology
11 November 2025
https://doi.org/10.1136/jitc-2025-012527
Systemic immunosuppression from ultraviolet radiation exposure inhibits cancer immunotherapy
31 October 2025
Institute Authors (4)
Isabella Mataloni, Antonia Banyard, Garry Ashton, Amaya Virós
Labs & Facilities
Mass and Flow Cytometry, Histology
Research Group
Skin Cancer & Ageing
31 October 2025
https://aacrjournals.org/cancerdiscovery/article/doi/10.1158/2159-8290.CD-24-1224/766638/Glucocorticoids-Unleash-Immune-dependent-Melanoma
Glucocorticoids Unleash Immune-dependent Melanoma Control through Inhibition of the GARP/TGF β Axis
15 October 2025
Institute Authors (12)
Charles Earnshaw, Poppy Dunn, Shih-Chieh Chiang, Maria Koufaki, Massimo Russo, Kimberley Hockenhull, Erin Richardson, Anna Pidoux, Alex Baker, Richard Reeves, Robert Sellers, Sudhakar Sahoo
Labs & Facilities
Computational Biology Support, Visualisation, Irradiation and Analysis
Research Group
Cancer Inflammation and Immunity
15 October 2025
/wp-content/uploads/2025/09/Annual_Report_2024.pdf
2024 Annual Report
23 September 2025
23 September 2025
https://doi.org/10.1182/blood.2024028033
An in vivo barcoded CRISPR-Cas9 screen identifies Ncoa4-mediated ferritinophagy as a dependence in Tet2-deficient hematopoiesis
4 September 2025
Institute Authors (1)
Justin Loke
Research Group
Myeloid Cancer Biology
4 September 2025
https://doi.org/10.1038/s41467-024-49692-1
Whole genome sequencing refines stratification and therapy of patients with clear cell renal cell carcinoma
15 July 2025
Institute Authors (1)
Samra Turajlić
Research Group
Cancer Dynamics
15 July 2025
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.


















A note from the Group Leader – Amaya Virós
My research is focused on studying why some melanomas are more metastatic. I am particularly keen to understand how cancer cells travel from the primary site to metastatic organs, and why some organs are more likely to be seeded by metastatic cells than others. We are focusing on the lungs, liver and brain as primary sites of metastasis. After decades of clinical experience and research, we still don’t fully understand why some patients have a more aggressive course of disease. We investigate local factors in the skin that promote metastasis, as well systemic and organ factors that allow melanoma cells to spread and thrive in a new environment. Understanding why cancer spreads will allow us to develop new agents to stop melanoma deaths.