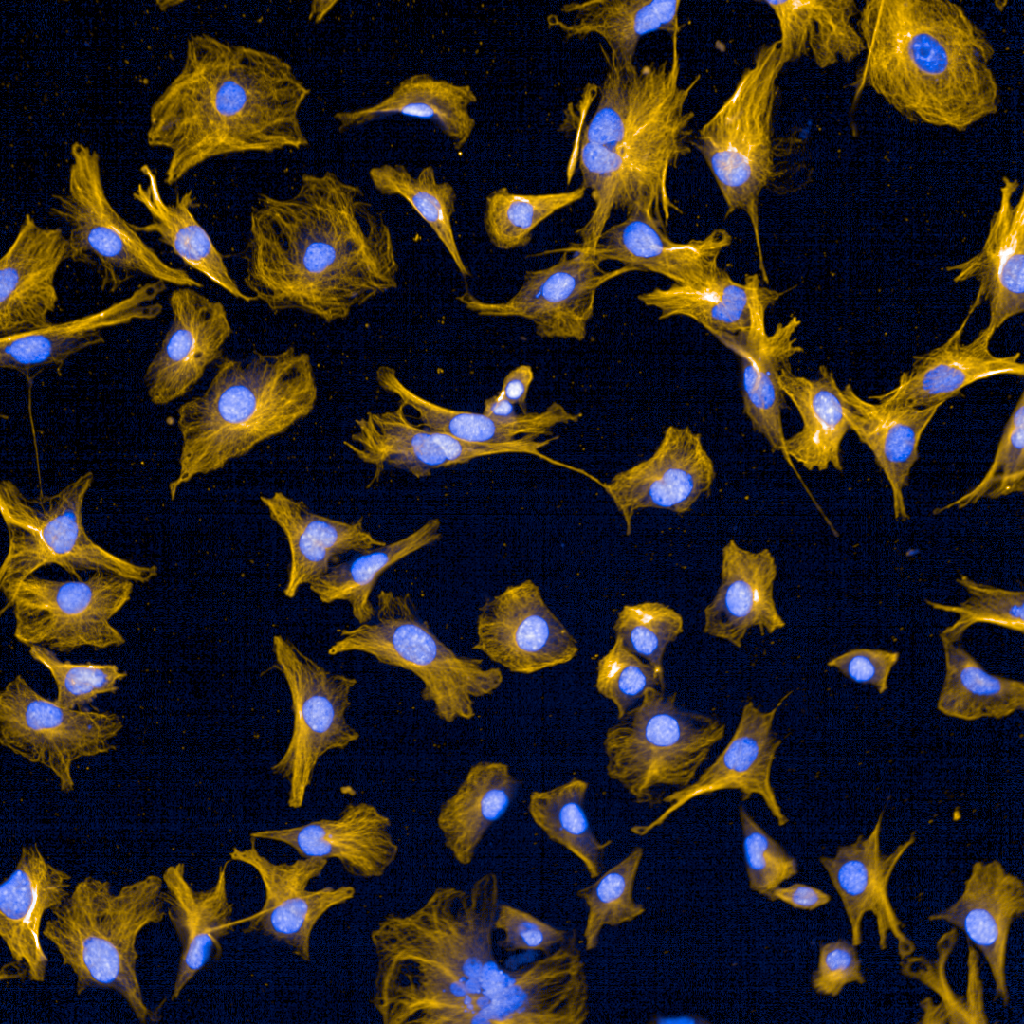
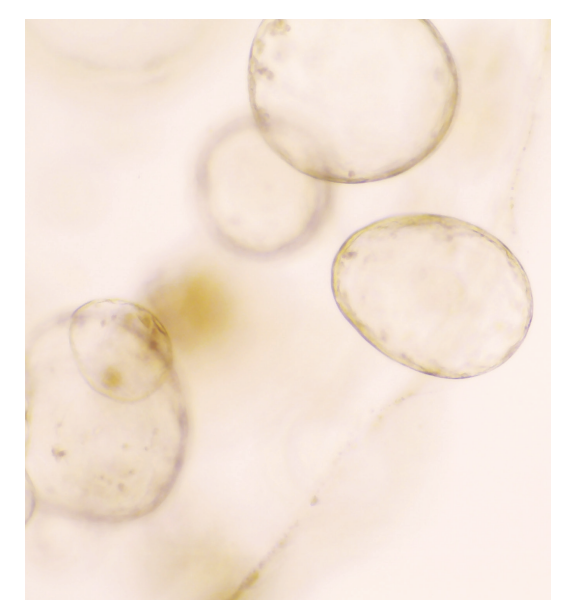
Overview
Tumour cells are embedded in a biophysically stiff environment and constitute less than 15% of the tumour volume in patients, yet most in vitro models do not replicate these aspects. In an ongoing collaboration with Prof Linda Griffith (MIT) and Prof Martin Humphries (UoM) we have adapted a fully synthetic scaffold to support the growth of both tumour and host cells. Peptide ligands were used to mimic adhesive signals found in the tumour microenvironment of pancreatic cancer, which enabled growth of both normal and tumour cells. Moreover, tumour cells grown in these scaffolds produce their own extracellular matrix, which we found engages integrin ligands in a similar manner to what is observed in vivo.
Due to the synthetic nature of these scaffolds, they can be modified to recapitulate the entire stiffness range of patient tumours. Notably, tumour cells exhibit different growth morphologies and signalling depending on the scaffold stiffness, suggesting that incorporation of these models will be important to further address the impact of the environment on tumour cell function and to functionally interrogate stromal targeted therapies in patient derived models. Ongoing work in the laboratory is seeking to fully explore the application of these models to interrogate tumour cell dependencies.