Overview
PKMYT1 in Cdk1-Cyclin B regulation in checkpoint control
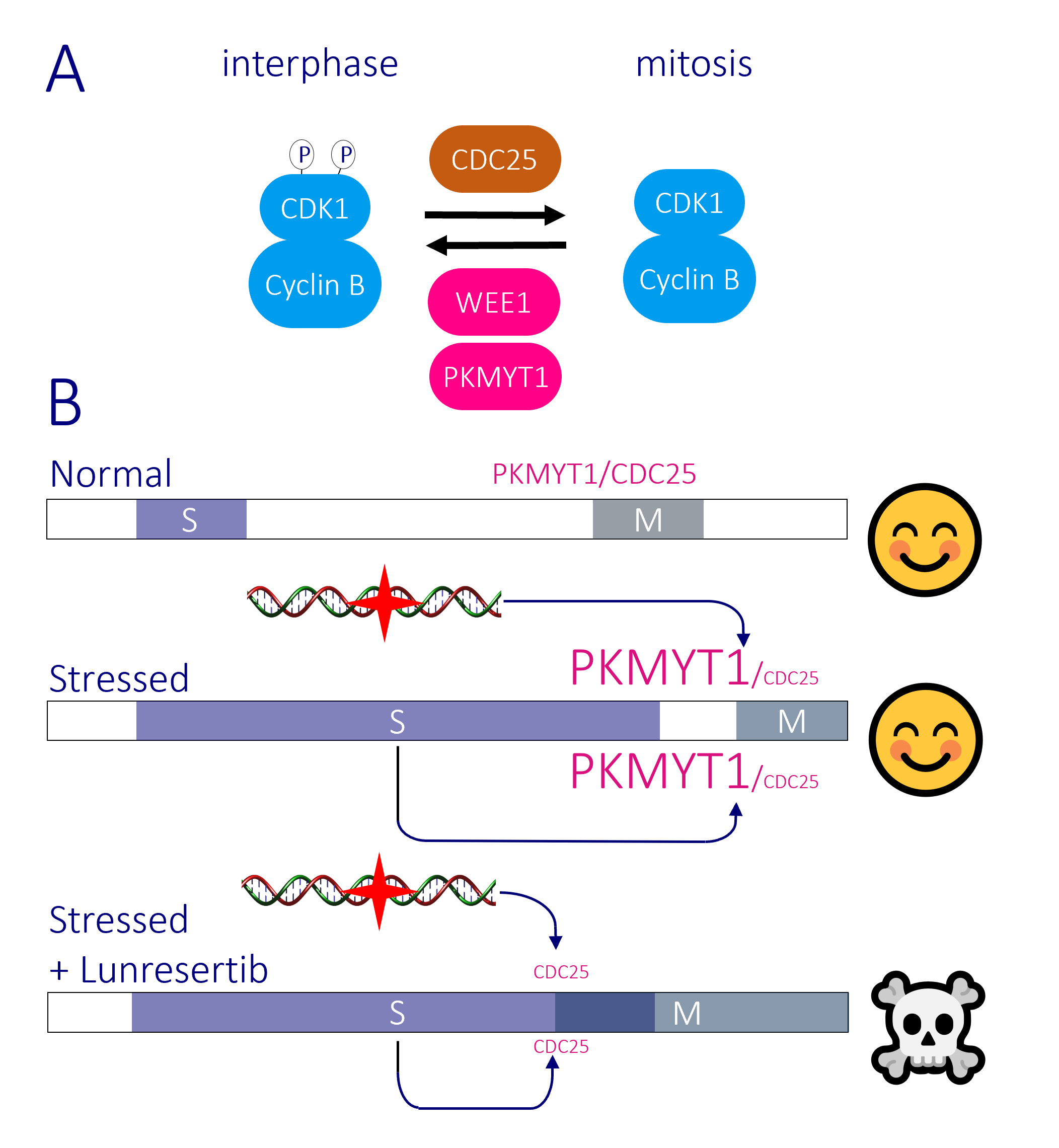
The transition from G2 phase into mitosis is driven by activation of the CDK1-Cyclin B protein kinase. CDK1-Cyclin B activity is restrained through inhibitory phosphorylation by the WEE1 family kinases WEE1 and PKMYT1. When the time is right, the inhibitory phosphate is removed by CDC25 phosphatases and cells enter mitosis. The checkpoint pathways that block mitotic commitment when DNA is damaged, or replication is incomplete, do so by boosting the activity of WEE1 family kinases and repressing CDC25 (Figure 2B). As cancer cells harbour more damage than normal tissue, they are more reliant upon these checkpoints than their normal neighbours, making targeting the checkpoint pathways a major focus in drug discovery at present.
WEE1 control of the CDK2-Cyclin Complexes that control the timing and execution of DNA replication alongside its inhibition of CDK1-Cyclin B means that clinical application of WEE1 inhibitors is proving problematical. In contrast, because PKMYT1 only regulates CDK1-Cyclin B and PKMYT1 can be completely removed from untransformed cells without affecting viability, the PKMYT1 inhibitor Lunresertib is generating great excitement as its excellent pre-clinical efficacy is being matched by performance with minimal toxicity in early clinical trials. We want to guide and refine the use of PKMYT1 inhibitors in the clinic by finding more about the basic biology of the molecule. We want to know how, when, and why PKMYT1 is used to regulate mitotic commitment.