Overview
Blood cancers often arise from mutations in genes that control normal stem cell development. Disorders originating in the bone marrow, such as myelodysplastic syndrome (MDS) and acute myeloid leukaemia (AML), provide valuable systems to study this process.
These diseases typically develop through the accumulation of multiple mutations in haematopoietic stem cells (HSCs), which give malignant cells a growth advantage over healthy ones. Over time, one dominant clone emerges, driving bone marrow failure and severe complications.
What remains poorly understood is how different mutations interact during cancer evolution and how these interactions shape disease behaviour.
Our lab uses advanced genetic editing techniques to build experimental models that more closely mimic the complex mutational landscapes seen in patients.
These models will help us uncover new biological insights and identify novel vulnerabilities that could guide the development of future therapies.
Featured Publications
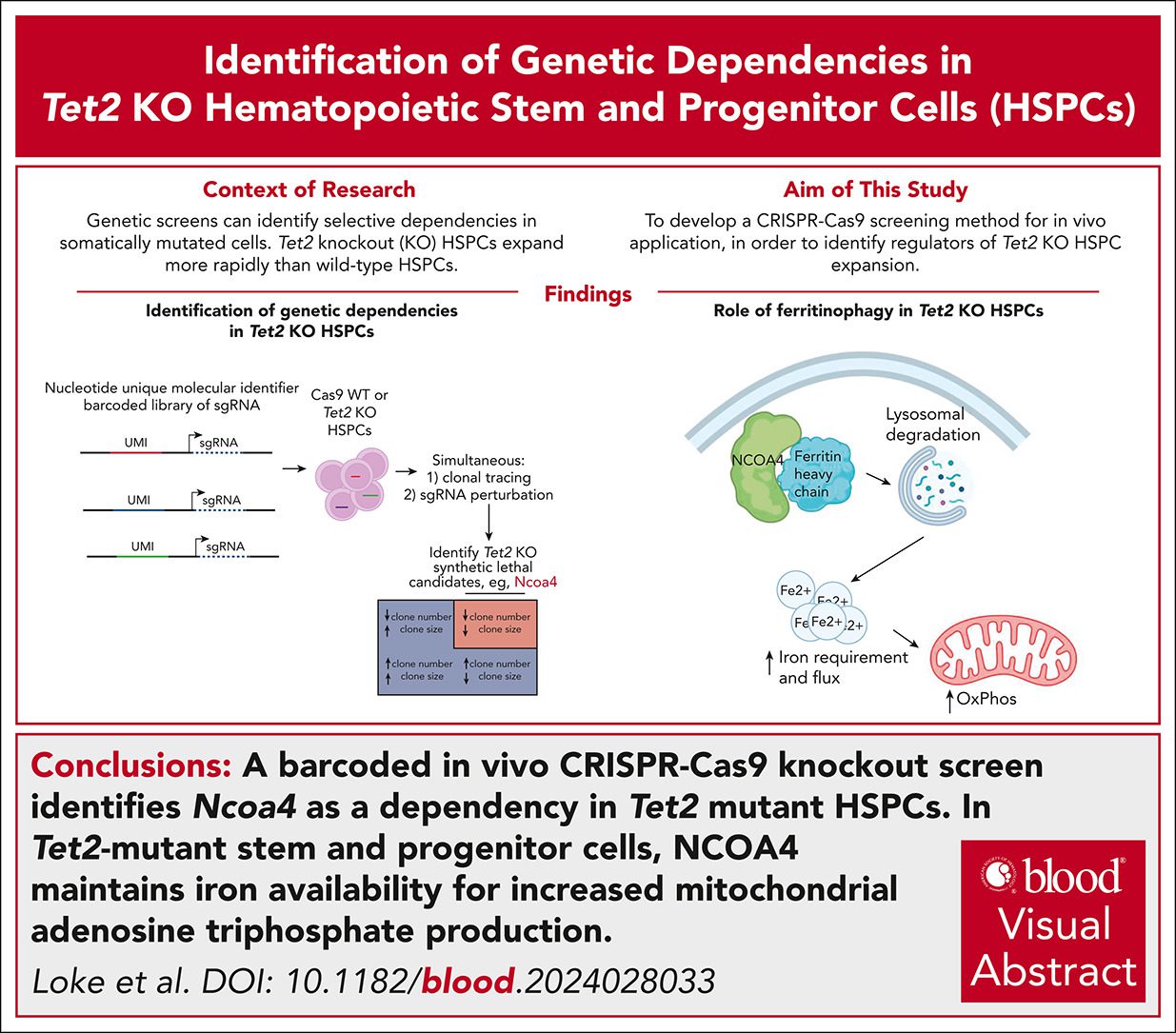
An in vivo barcoded CRISPR-Cas9 screen identifies Ncoa4-mediated ferritinophagy as a dependence in Tet2-deficient hematopoiesis
4th September 2025
Authors use barcoded in vivo CRISPR-Cas9KO screen to identify Ncoa4 as a dependency in Tet2-mutant HSPCs and reveals a promising therapeutic target for early intervention.
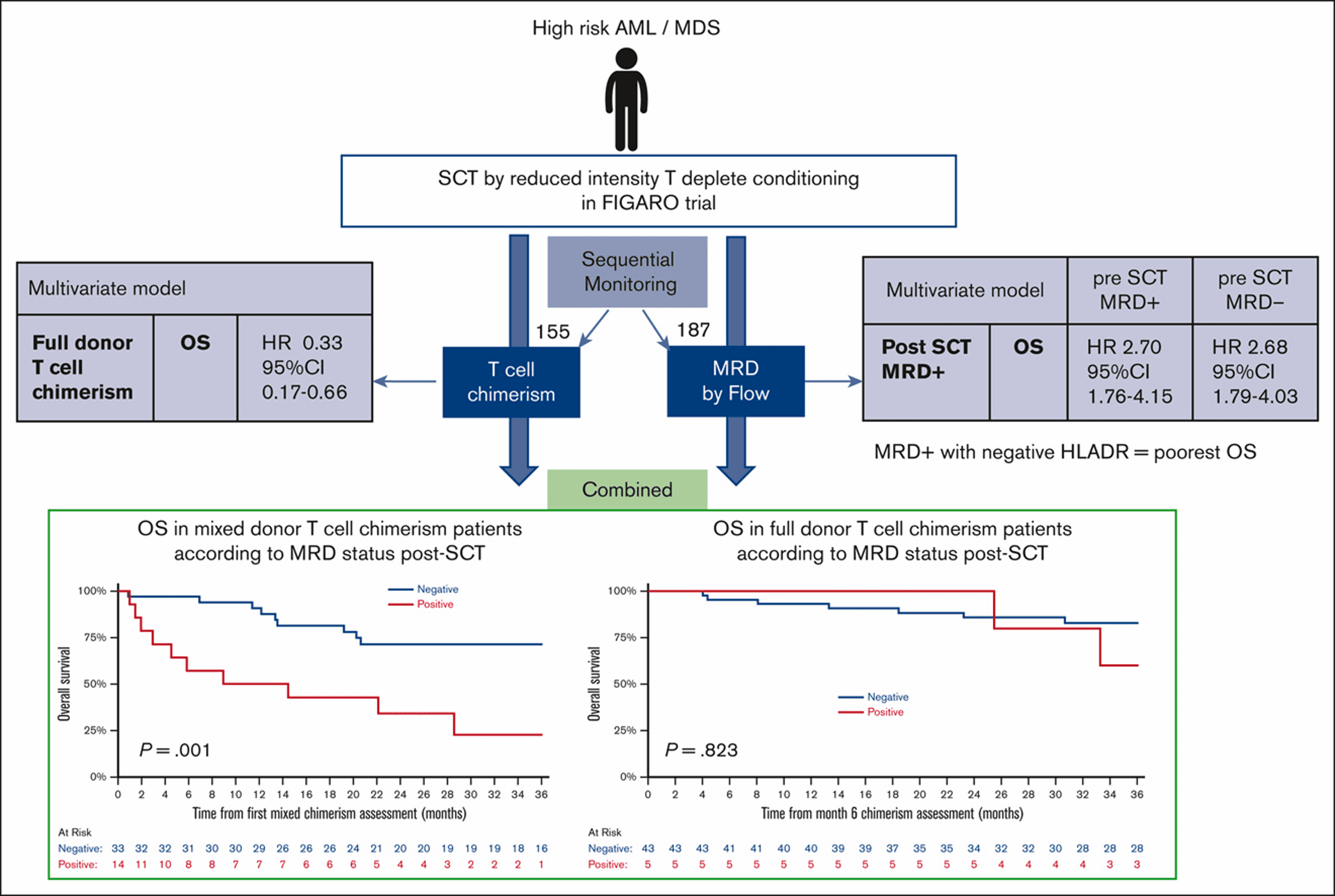
Posttransplant MRD and T-cell chimerism status predict outcomes in patients who received allografts for AML/MDS
17th July 2023
Authors find presence of posttransplant MRD has an adverse prognostic impact on the outcome irrespective of pretransplant MRD in patients with AML/MDS.
Additional cytogenetic features determine outcome in patients allografted for TP53 mutant acute myeloid leukemia
25th May 2022
Loke et al show presence of TP53 mutations in acute myeloid leukemia (AML) are associated with an inferior outcome after an allogeneic stem cell transplant.
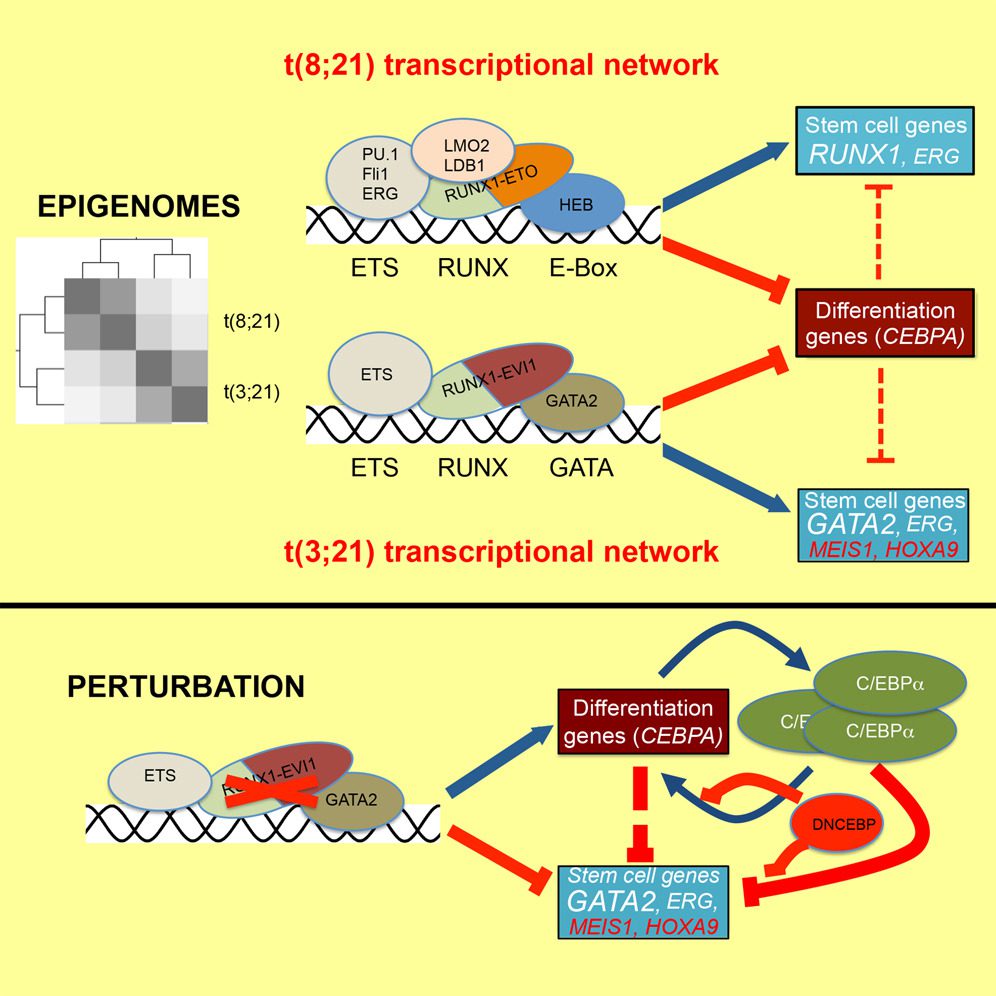
RUNX1-ETO and RUNX1-EVI1 Differentially Reprogram the Chromatin Landscape in t(8;21) and t(3;21) AML
23rd May 2017
Loke et al. compare the regulatory signature of two related types of acute myeloid leukemia, t(8;21) expressing RUNX1-ETO and t(3;21) expressing RUNX1-EVI1.
Recent research
In recent work led by Benjamin Ebert and colleagues from Dana-Farber Cancer Institute, we have shown that ferritinophagy, a selective autophagic process mediated by the cargo receptor Ncoa4, supports the expansion of Tet2-mutant hematopoietic stem and progenitor cells (HSPCs) by maintaining mitochondrial iron availability and oxidative metabolism. This work identified a novel metabolic dependency in Tet2-deficient clonal hematopoiesis and revealed a promising therapeutic target for early intervention.
Using a barcoded in vivo CRISPR-Cas9 KO screen, we identified the cargo receptor Ncoa4 as a dependency in Tet2-mutant haematopoietic stem and progenitor cells (HSPCs).
In Tet2-mutant stem and progenitor cells, NCOA4 maintains iron availability for increased mitochondrial adenosine triphosphate production.
FAQS
We are currently recruiting a clinical PhD position to join our lab. Deadline 17 November.
For further details, please go to FindAPhD.com
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.












A note from the Group Leader – Justin Loke
I am delighted and privileged to join Manchester with experience of new technologies and scientific training from the Dana-Farber Cancer Institute.
I believe the cutting-edge scientific environment at the Cancer Research UK Manchester Institute, integrated with the internationally renowned clinical expertise at The Christie, will enable the development of a translational research programme to discover new mechanisms driving aggressive blood cancers and improve treatments for our patients with these diseases.