Overview
The immune system constitutes a natural host defence mechanism to life-threatening insults. Although mainly studied in the context of infectious diseases, it is now precedented that the immune system can inhibit cancer formation by detecting and eradicating malignant cells. Cells of the adaptive immunity called T cells, which can act as immune killers, are often the spearhead of anti-cancer immunity. Although the success is limited to small fraction of cancer patients, T cell-based immunotherapies have dramatically improved clinical outcome in fatal cancers.
Instruction of T cell immunity is a key function of dendritic cells (DC). Like other innate immune sensor cells, DC express a wide repertoire of receptors endowing them with the ability to detect microbial presence and tissue damage. How these functions contribute to anti-cancer immunity remains unclear but dying cancer cells and certain commensal bacterial taxa have been linked to induction of T cell-mediated anti-cancer immunity and enhanced responses to immunotherapy. Deciphering the mechanisms that link the gut microbiome and dying cancer cells to effective T cell immunity is of paramount importance to overcome intrinsic and acquired resistance to cancer immunotherapy.
Scientific interest
In the Cancer Immunosurveillance group, we combine genetically altered mouse models, modified diets as well as engineering of commensal microbes and tumours to disentangle complex microbiome-host-tumour interactions that underpin cancer immunity. We focus on two fundamental pillars of host physiology, cell death and microbes, that trigger and shape adaptive anti-cancer immune responses under the umbrella of innate immunity. Using our previously and newly established models, we aim to characterise the mechanisms that couple host sensing of (1) commensal microbes and/or (2) damaged cells to T cell-mediated cancer immunity. Our ultimate vision is to contribute to the basic understanding of cancer immunity and pave the way for therapeutic approaches to overcome immunotherapy resistance.
Featured Publications
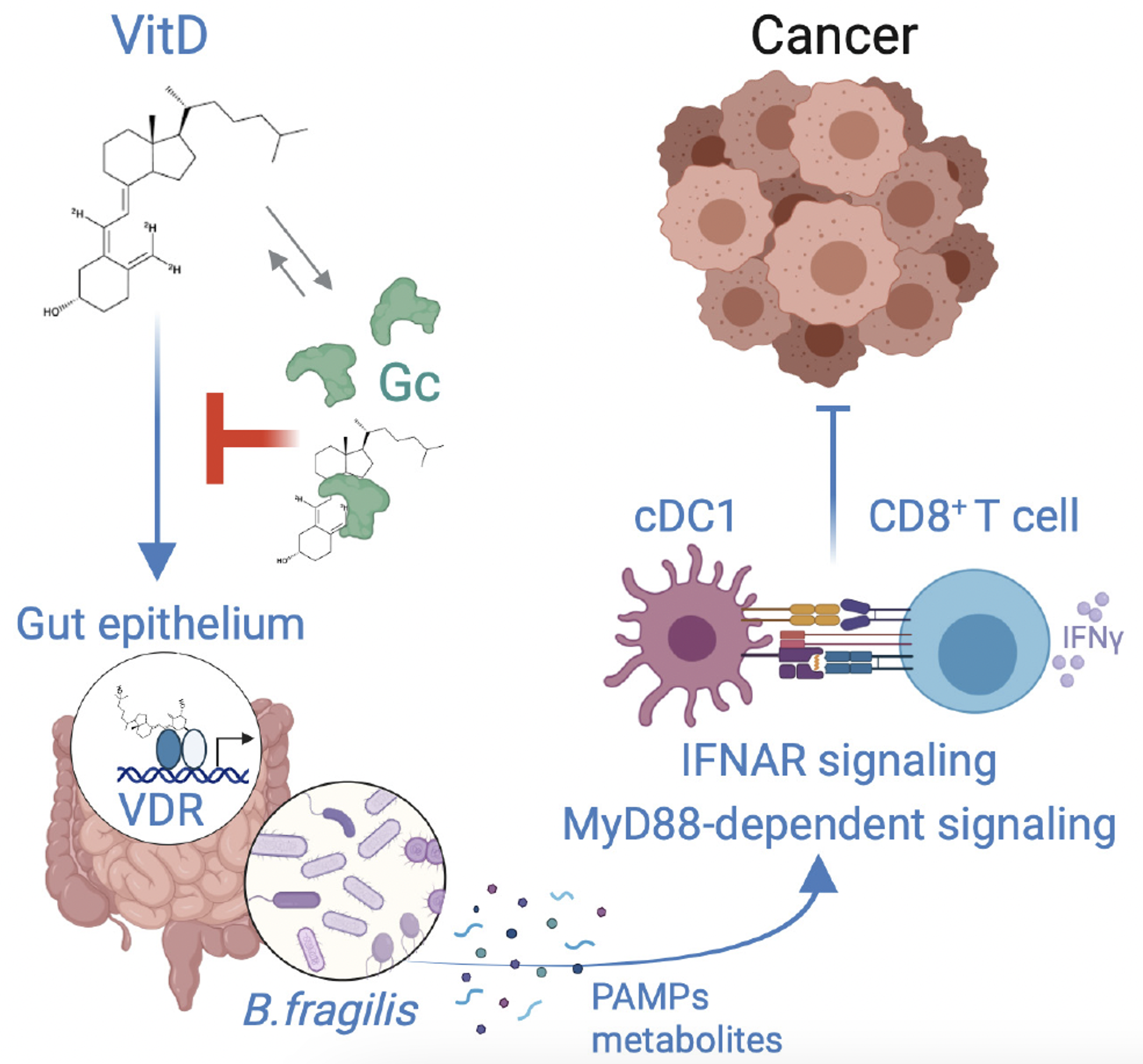
Vitamin D regulates microbiome-dependent cancer immunity
25th April 2024
The gut microbiome has been shown to modulate the response of cancer patients to therapy, but precisely how microbiota affect anticancer immunity is still being elucidated. Giampazolias et al. report that vitamin D bioavailability in mice influences the composition of the gut microbiome.
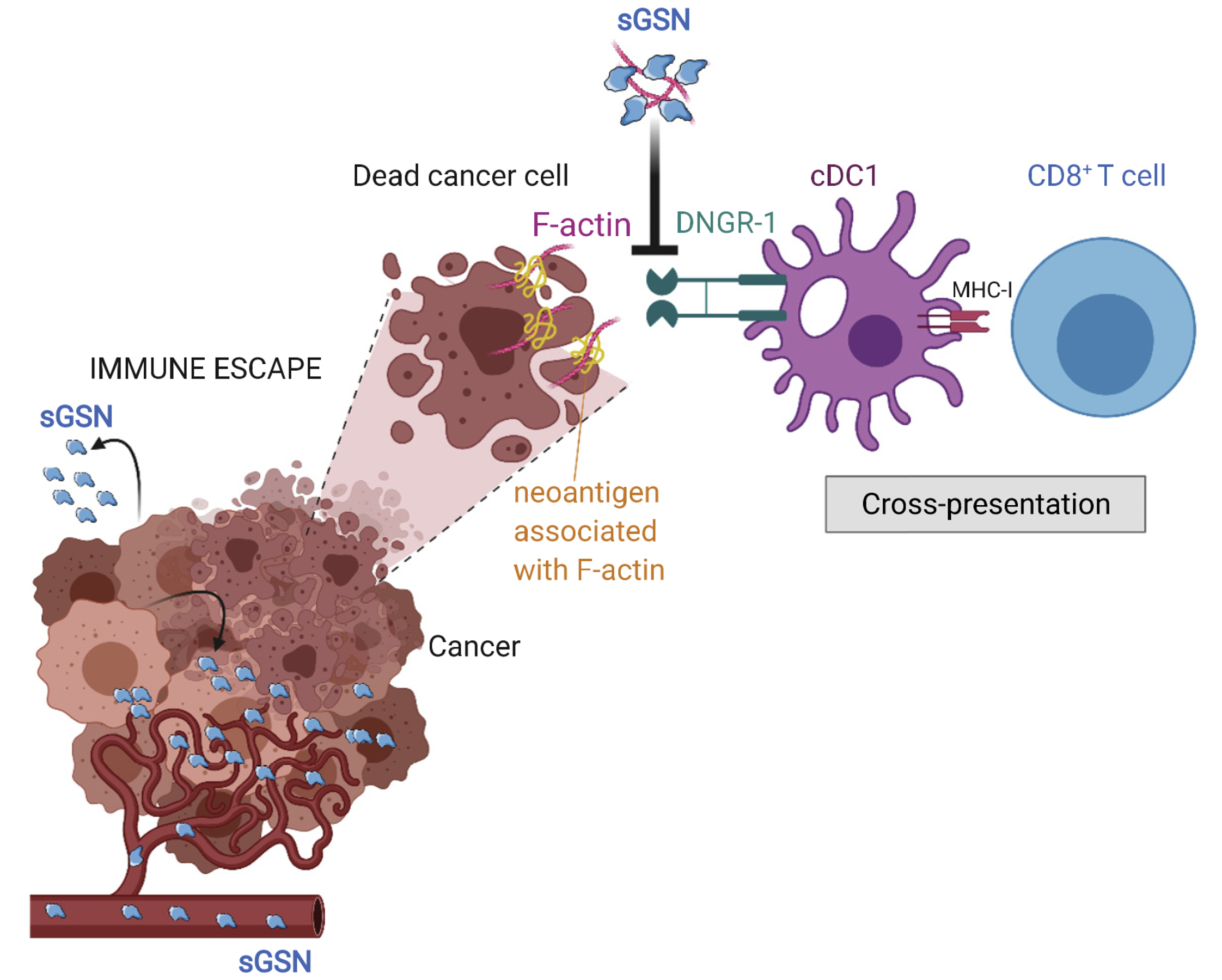
Secreted gelsolin inhibits DNGR-1-dependent cross-presentation and cancer immunity
2nd June 2021
The secreted gelsolin component of the plasma actin-scavenging system impairs the ability of the receptor DNGR-1 to recognize dead cells and selectively dampens cross-presentation of tumor antigens by type 1 dendritic cells, acting as a barrier to anti-tumor immunity.
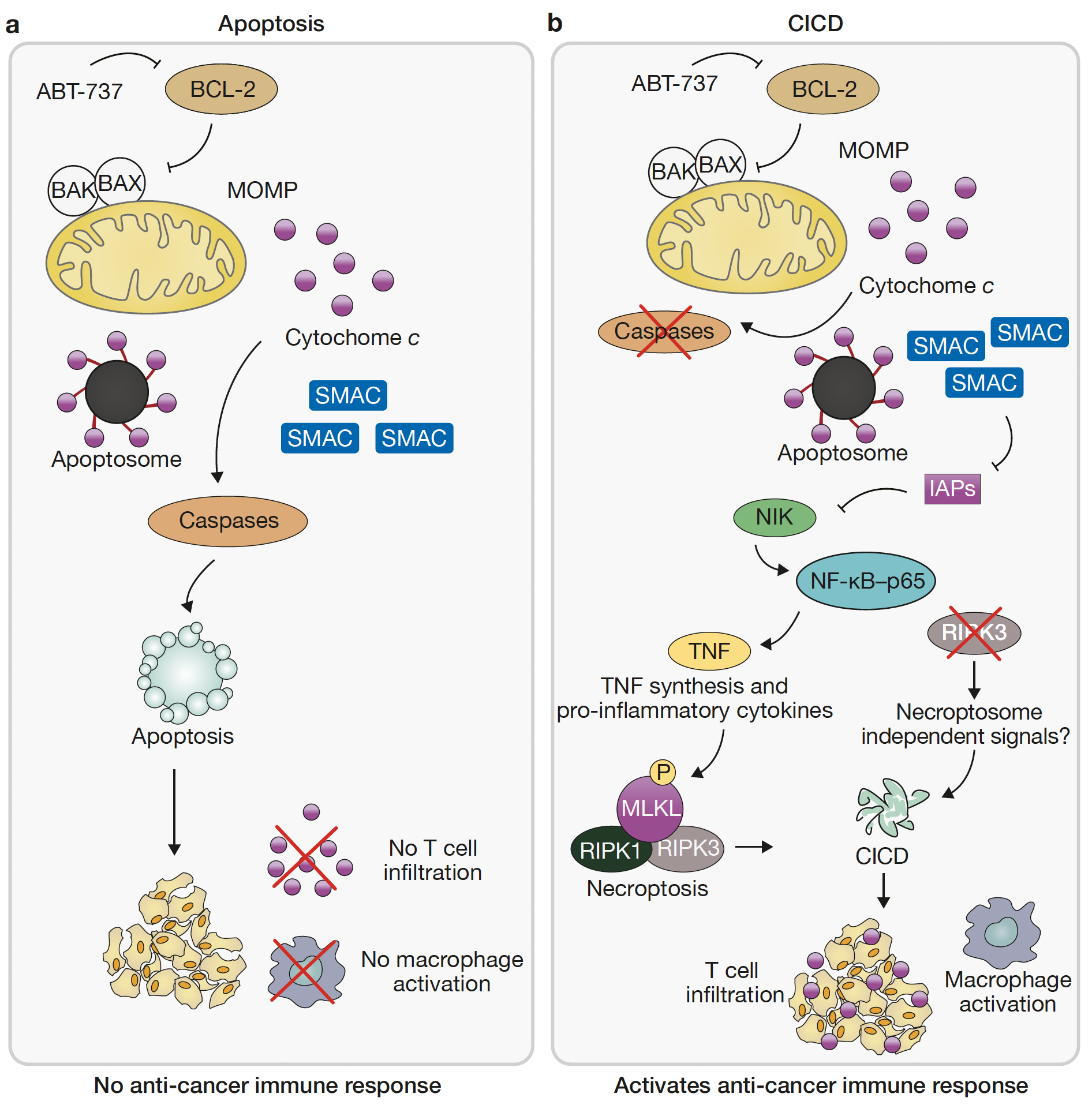
Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency
28th August 2017
Authors show that activating NF-κB allows MOMP to exert additional signalling functions besides triggering cell death. Findings support a rationale for engaging caspase-independent cell death in cell-killing anti-cancer therapies.
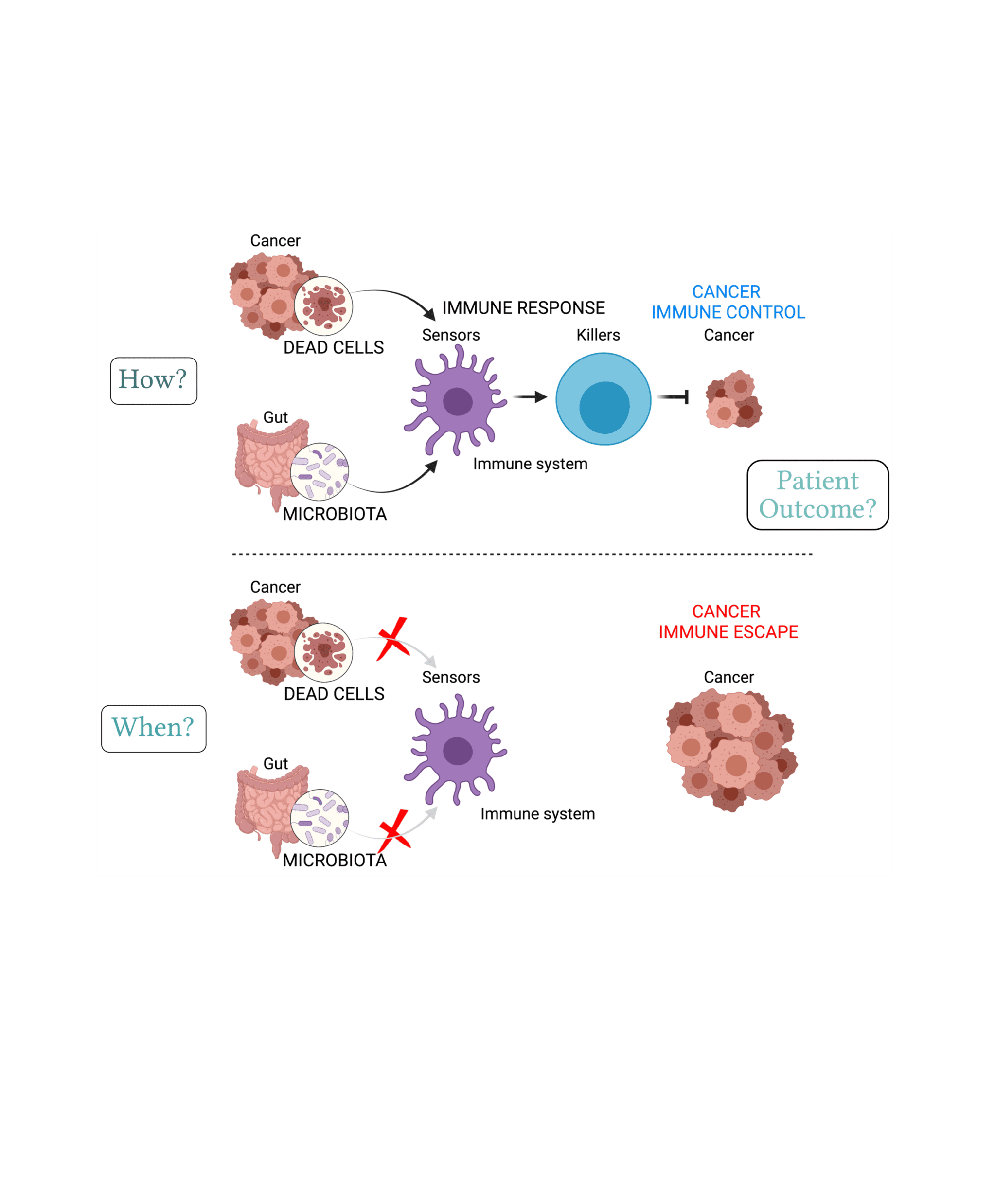
Research overview
Host detection of signals derived from dead cells and commensal microbes trigger cancer immunity.
BBC Science Focus interview
“We were really intrigued. We wanted to find this magic trait of cancer resistance that can be passed from one mouse to the other. One of the main things that came to our minds is that faecal matter is a big source of communities of friendly microorganisms that live inside the gut.”
Read more from this BBC Science Focus interview with Evangelos on the relationship between Vitamin D, the gut microbiome and cancer immunity in our recent blog post: Institute Group Leader in BBC Interview on Cancer Immunity
FAQs
We are currently recruiting a PhD studentship position in our group, please see details of our available project and how to apply – deadline 28 November 1700 hrs.
We are looking for enthusiastic postdocs to study nutrient-host-microbiome interactions that define immunity to cancer in mice and humans. If you are interested in our work, please feel free to get in touch directly using the contact form below.
Get in touch
Our vision for world leading cancer research in the heart of Manchester
We are a leading cancer research institute within The University of Manchester, spanning the whole spectrum of cancer research – from investigating the molecular and cellular basis of cancer, to translational research and the development of therapeutics.
Our collaborations
Bringing together internationally renowned scientists and clinicians
Scientific Advisory Board
Supported by an international Scientific Advisory Board
Careers that have a lasting impact on cancer research and patient care
We are always on the lookout for talented and motivated people to join us. Whether your background is in biological or chemical sciences, mathematics or finance, computer science or logistics, use the links below to see roles across the Institute in our core facilities, operations teams, research groups, and studentships within our exceptional graduate programme.


















A note from the Group Leader – Evangelos Giampazolias
My research interest is focused on understanding the mechanisms that makes cancer a visible threat to the immune system. Specifically, I study the ‘dialogue’ between gut commensals or damaged cells with host cells and how these interactions lead to cancer immunity.
In my group, we work with in vivo mouse models and gut organoids using spectral flow cytometry, microscopy, single-cell or bulk transcriptomics, proteomics and metabolomics in host cells or commensal species to answer both unbiased and hypothesis-driven objectives that will allow us to identify instructive factors of cancer immunity. In collaboration with oncologists at the Christie Hospital we have developed a protocol of biospecimen collection that will allow us to translate our basic findings to the clinic by assessing the predictive value of nutrient-host-microbiome interactions in cancer patient outcome and immunotherapy response.
Through our interdisciplinary background, established international collaborations and cutting-edge facilities of CRUK Manchester institute, we continue to identify novel mechanisms of immune defence that will contribute to our general understanding on how the immune system respond to insults and might pave the way for developing new immunotherapeutic approaches.